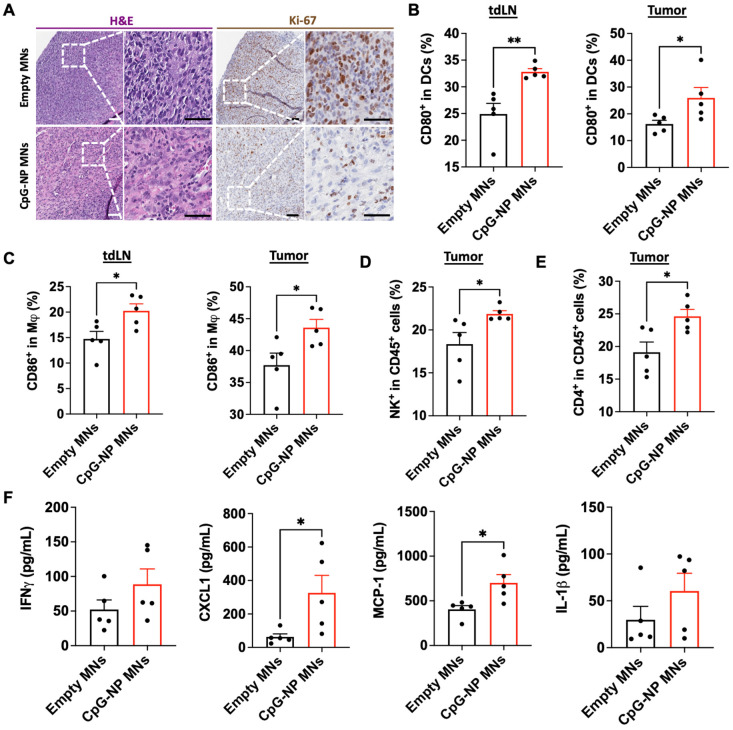
Figure 5.
Delivery of CpG-NPs using MNs modulates the immune composition in the TME. A, Representative H&E staining (left, scale bar: 100 μm; right: scale bar: 50 μm) and Ki-67 staining (left, scale bar: 100 μm; right: scale bar: 50 μm) of MC38 tumors harvested on day 20 post-tumor induction (n = 5). B, Flow cytometry quantification of activated dendritic cells (CD80hi CD11c+MHCII+CD45+) in tdLNs (left) and tumors (right) 48 h post transdermal delivery of CpG-NPs. Data are mean ± s.e.m. (n = 5). C, Flow cytometry quantification in the tdLNs (left) and tumors (right) of activated macrophages (CD86hi F4/80+CD11b+CD45+) 48 h following MN-mediated delivery of CpG-NPs. Data are mean ± s.e.m. (n = 5). D-E, Flow cytometric quantification of the percentage of natural killer cells (d) and CD4+ cells (e) in tumor lysates 48 h post-treatment with CpG-NPs. F, Analysis of the pro-inflammatory cytokine/chemokine profile in tumors using a bead-based immunoassay (Legendplex™). Data are mean ± s.e.m. (n = 5). ***P < 0.001, **P < 0.01, *P < 0.05. Data were analyzed by Grubbs' test for statistical outliers, which were pre-defined using an alpha value of 0.01.