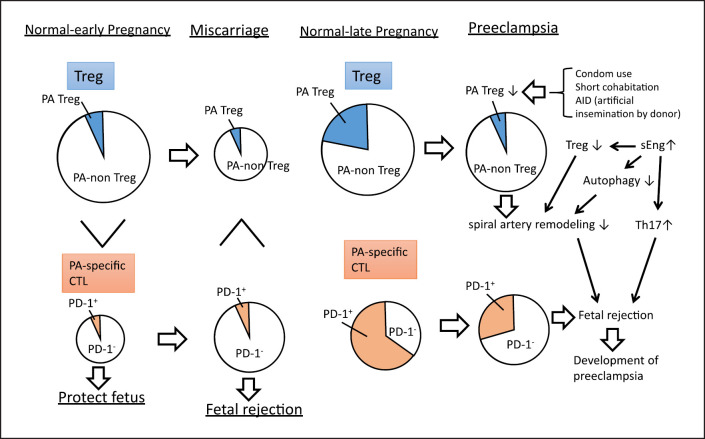
Fig. 2.
Changes in regulatory T (Treg) cells in miscarriage and preeclampsia. The size of the circle represents the cell numbers. The frequencies of paternal antigen-specific Treg (PA-Treg) and PA-nonspecific Treg (PA-non-Treg) among Treg cells, and PD-1+ cytotoxic T (PD-1+CTL) and PD-1−CTL among CTL are shown. The total number of Treg cells in miscarriage is lower than that in healthy early pregnancy, while the percentage of paternal antigen-specific Treg cells remains unchanged. Compared with that in healthy early pregnancies, the number of paternal antigen-specific cytotoxic T cells is higher in miscarriage. In the early pregnancy period, PD-1− paternal antigen-specific cytotoxic T cells are predominant, resulting in fetal rejection. In late normal pregnancy, the total numbers of Treg cells and paternal antigen-specific Treg cells are upregulated. In preeclampsia, there is not only a decrease in Treg volume, but also a decrease in PA-Treg cell rate. As shown in the upper right of the figure, in cases of inadequate semen exposure, such as condom use, short cohabitation, and sperm donor pregnancies, mothers are at risk for preeclampsia due to insufficient induction of paternal antigen-specific Treg cells. In addition to the decrease in Treg cell volume and ratio of paternal antigen-specific Treg cells to nonpaternal antigen-specific Treg cells, increased secretion of soluble endoglin (sEng) may suppress the autophagy of endovascular trophoblasts (EVTs) in preeclampsia (middle right panel). This results in impaired remodeling of spiral arteries. In addition, sEng neutralizes TGF-β, thus inhibiting the differentiation into Treg cells and promoting differentiation into inflammatory Th17 cells (middle right panel). Additionally, PD-1 expression in paternal antigen-specific CTLs is downregulated, and the fetus may be attacked by maternal cytotoxic T cells, contributing to the development of preeclampsia (lower right).