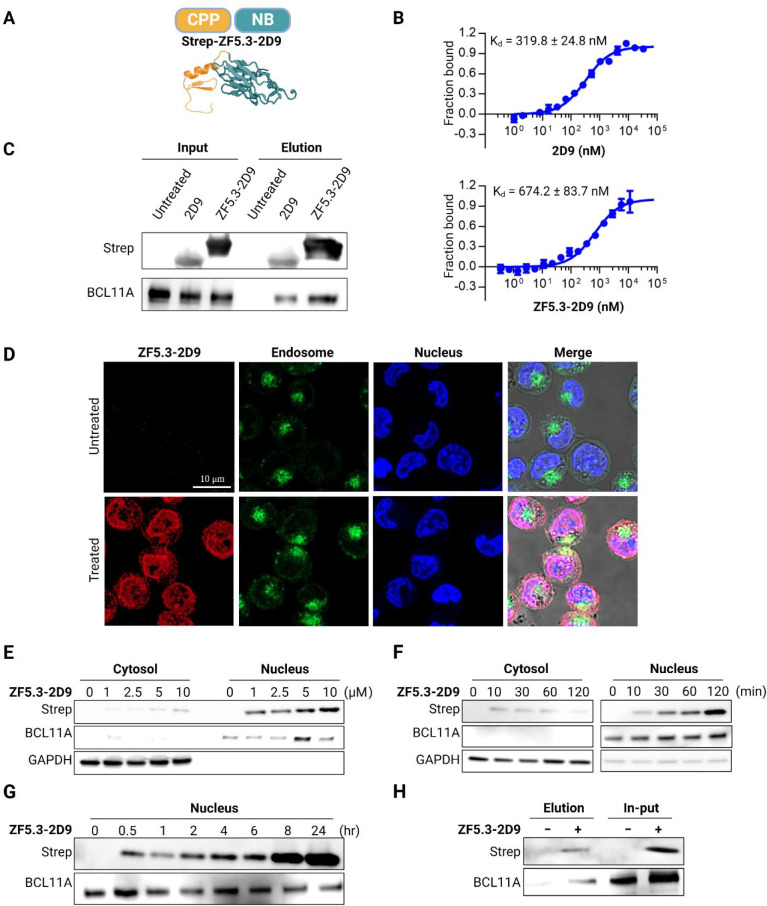
Figure 2.
Delivery of ZF5.3-2D9 to erythroid precursor cells. (A) Schematic description of the cell-permeant Nb ZF5.3-2D9 created from the individual structures of ZF5.3 (modeled secondary structure) and 2D9 (PDB 7UTG). (B) MST analysis of the binding of 2D9 and ZF5.3-2D9 to exZnF23 of BCL11A (mean ± SD, n = 3). (C) Immunoprecipitation revealing that 2D9 and ZF5.3-2D9 bind to endogenous BCL11A. (D) Confocal microscopy images of HUDEP-2 cells revealing ZF5.3-2D9 (red) entered HUDEP-2 cells and show significant colocalization (purple) with the nucleus (blue). Immunoblots revealing a (E) concentration- and (F) time-dependent cell penetration by ZF5.3-2D9. (G) Immunoblot showing increased accumulation of ZF5.3-2D9 in the nucleus. (H) Co-immunoprecipitation of endogenous BCL11A using delivered ZF5.3-2D9. GAPDH and BCL11A were used as loading controls for the cytosolic and nuclear fractions, respectively.