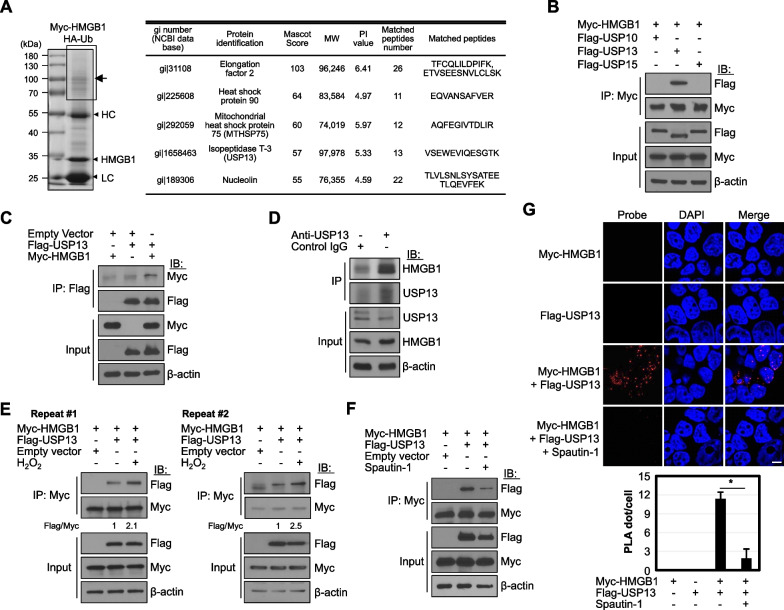
Fig. 1.
USP13 interacts with HMGB1. A HEK293T cells were co-transfected with both Myc-HMGB1 and HA-Ub plasmids for 48 h, and then treated with 10 μM MG132 for the last 18 h prior to harvest. WCLs were immunoprecipitated with anti-Myc affinity gel and separated by SDS-PAGE. Indicated box (arrow) was extracted for LC-MS/MS analysis. HC and LC: heavy and light chains. HMGB1 binding proteins were identified using LC-MS/MS for the analyses of peptides. B HEK293T cells were transfected with both Myc-HMGB1 and Flag-tagged USP10 or USP13 or USP15 plasmids and cultured for 48 h. WCLs were immunoprecipitated (IP) and immunoblotted (IB) with the indicated antibodies. C HEK293T cells were immunoprecipitated with anti-Flag antibody and immunoblotted with the indicated antibodies. D HEK293T cells were immunoprecipitated with anti-USP13 antibody, and the binding of endogenous HMGB1 was shown. E, F Both Myc-HMGB1 and Flag-USP13 were overexpressed in HEK293T cells, and cells were treated with 50 μM H2O2 E or 10 μM Spautin-1 F for the last 18 h before harvesting them to observe the binding. Data represents one of two similar independent experiments in E. G PLA was performed on HEK293T cells co-expressing Myc-HMGB1 and Flag-USP13 with 10 μM Spautin-1 treatment for last 18 h. For the PLA spot analysis, over 20 cells were counted. Scale bar: 10 μm. *p < 0.001 using Tukey’s honestly significant difference post-hoc test for multiple comparisons