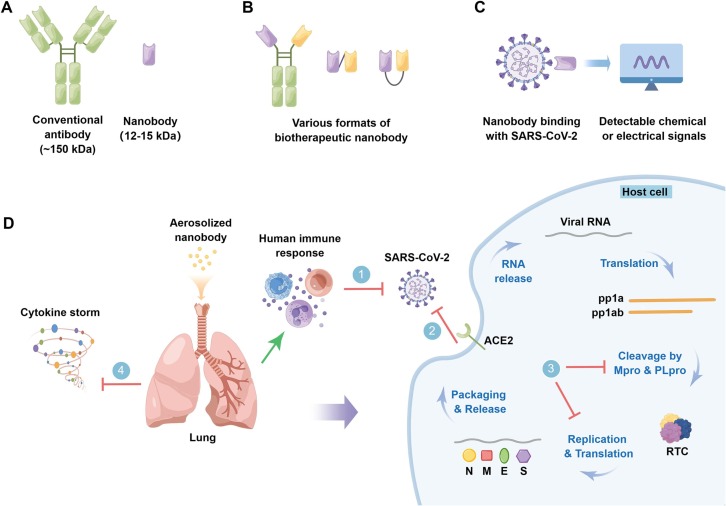
Fig. 2.
Prevention, Detection, and treatment of the SARS-CoV-2 and its variants with nanobodies. A. Schematic representation of conventional antibody and nanobody which is the variable domain of heavy chain-only antibody (HCAb) and only one tenth the size of the former. B. Nanobodies are adaptable to different multivalent formats, enabling enhanced avidity, simultaneous engagement of multiple spatially discrete non-overlapping epitopes, and mitigation of viral escape, especially when using conserved epitopes as targets. Here, multivalent formats of nanobodies are exemplified by dimerization achieved by genetic immunoglobulin (Ig) Fc fusion or C-to-N-terminal assembly, or otherwise C-to-C-terminal fusion of nanobodies through chemical approach. C. Application of robust nanobodies in SARS-CoV-2 detection, especially in point-of-care testing (POCT), during which binding events are translated into detectable chemical or electrical signals. D. Local pulmonary delivery of aerosolized nanobodies is promising in prevention and treatment of the SARS-CoV-2 and its variants by inducing human immune response (1), blocking their life cycle at different stages, such as viral entry (including viral binding and the subsequent membrane fusion) (2) and viral replication (3), or by modulating host inflammation (cytokine storm) (4). Green line with arrow indicates “Induction”, and red “T” shaped lines indicate “Inhibition”. By Figdraw.