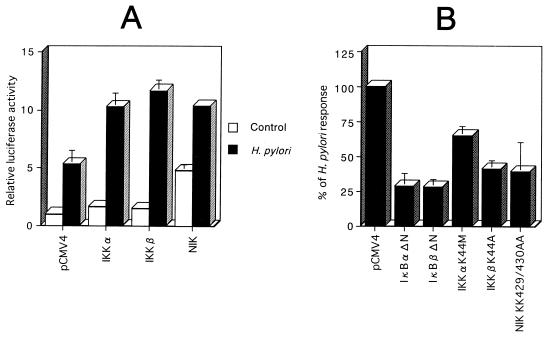
FIG. 4.
Functional analysis of the roles of IKK-α, IKK-β, and NIK in H. pylori-induced activation of the MCP-1 distal enhancer. (A) Functional effects of wild-type IKK-α, IKK-β, and NIK on H. pylori-induced activation of the MCP-1 distal enhancer. MKN45 cells were transfected with 1 μg of pGLM-ENH and 5 μg of the wild-type IKK-α, IKK-β, or NIK expression vector. Each transfection also contained 2 μg of pRL-TK and was supplemented to 5 μg with pCMV4 vector. Twenty-four hours after transfection, half of the transfectants were cultured with H. pylori for 6 h, while the other half were left untreated. The cells were lysed and assayed for luciferase activity. Luciferase activity is presented as fold induction relative to the basal level measured in cells transfected with pCMV4 without further treatment. (B) Functional effects of IκB-α and IκB-β dominant interfering mutants and kinase-deficient IKK-α, IKK-β, and NIK mutants on H. pylori-induced activation of the MCP-1 distal enhancer. MKN45 cells were transfected with 1 μg of pGLM-ENH and 5 μg of the IκB-α mutant IκB-αΔN, IκB-β mutant IκB-βΔN, the K44M mutant of IKK-α, the K44A mutant of IKK-β, or the kinase-deficient KK429/430AA mutant of NIK and then infected with H. pylori for 6 h. All values were first calculated as fold induction relative to cells transfected with pCMV4 without further treatment. These values were then expressed as percentages of the response obtained with H. pylori infection. Data represent means ± standard deviations from three independent experiments.