Fig. 2.
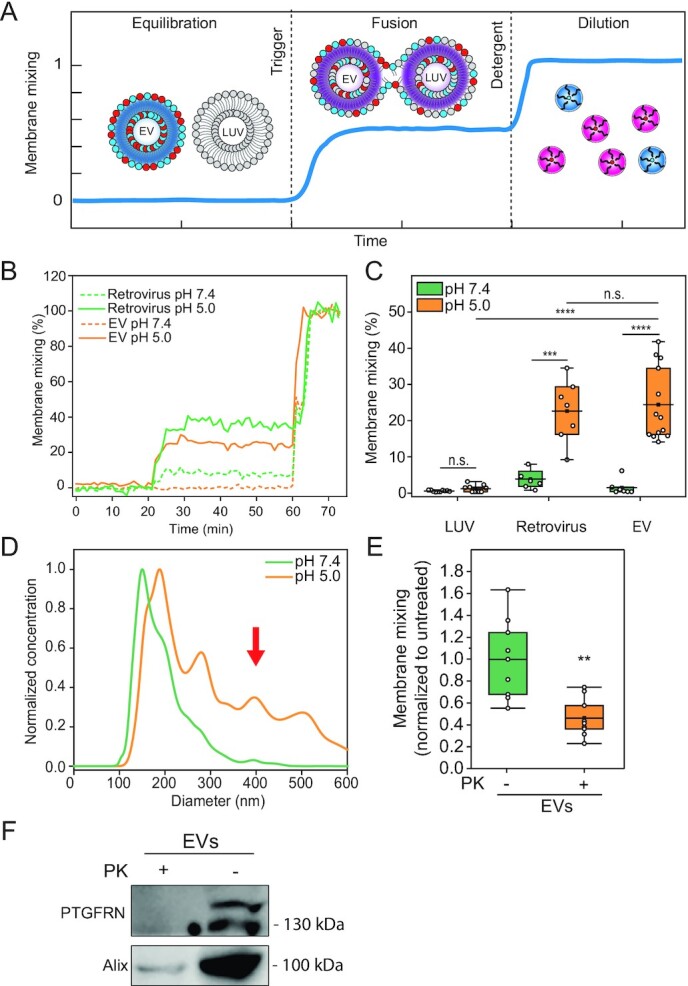
EV fuse in a protein- and pH-dependent manner reminiscent of viruses. (A) Illustration of the FRET-based membrane mixing assay employed to quantify fusion between EVs and liposomes. Highly FRET-efficiency labeled EVs are incubated with nonlabeled liposomes and their ability to fuse is probed by monitoring the donor fluorescent intensity after triggering. (B) Representative curves of membrane mixing assay for either retrovirus (green) or EV (orange) incubated with unlabeled LUVs at pH 7.4 (dotted line) or pH 5.0 (solid line). (C) FRET fusion assay for labeled retroviruses, EVs and LUVs, incubated with LUVs at either pH 7.4 (green) or pH 5.0 (orange), showing that retroviruses and EVs fuse with similar efficiencies. LUVs mimicked the late endosome lipid composition. (D) Representative NTA size distribution curves of EV–LUV mixtures upon incubation at pH 7.4 (green) or pH 5.0 (orange) showing an increase in vesicle size after mixing and triggering with pH 5.0. (E) FRET membrane mixing assay comparing EVs treated with proteinase K (PK, orange) or nontreated (green), incubated with late endosomal-mimicking LUVs at pH 5.0. (F) Western blot for membrane protein EV marker, PTGFRN, and intraluminal protein Alix for nontreated and PK-treated EVs, showing that proteinase only digests proteins on the surface of the EVs.
