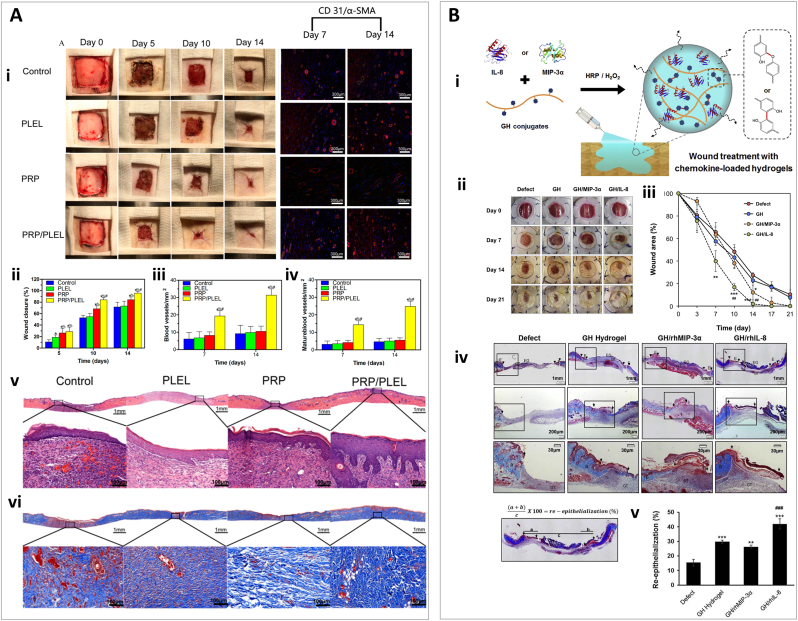
Fig. 6.
Wound healing using growth factor-containing hydrogels. (A) (i) Left: Images of full-thickness wounds treated with Poly (D, l-lactide)-poly (ethylene glycol)-poly (D, l-lactide) (PLEL) Platelet-rich plasma (PRP), PRP/PLEL or left untreated at days 0, 5, 10, and 14. Right: Immunofluorescent staining for CD31 and α-SMA shows newly formed blood vessels. (ii) Wound closure rate showing the best performance by PRP/PLEL hydrogels. (iii) Quantification of new blood vessels. (iv) Quantification of mature blood vessels. Hematoxylin and eosin H&E (v) and MT (vi) staining of wounds treated with PLEL, PRP, PRP/PLEL and controls showed better tissue maturation with PRP/PLEL hydrogels. Reprinted and adapted with permission from Qiu et al., 2016 [151]. (B) Hydrogels loaded with chemokines. (i) Schematic illustration of sprayable gelatin hydrogels (GH) loaded with chemokines (IL-8 or MIP-3α) for wound healing. (ii) Pictures of wounds treated with chemokine-loaded GH in STZ-induced diabetic mice on days 0, 7, 14, and 21. (iii) Wound closure rates are presented as a percentage of the initial wound area at day 0. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. defect; ##p < 0.01 vs. GH hydrogel. (iv) Masson's trichrome (MT) stain that shows re-epithelialization of the wounds treated with GH, GH containing MIP-3a or IL-8. D, dermis; E, epidermis; EG, epithelial gap; GT, granulation tissue. (v) Re-epithelialization percentage between treatments and control groups. **p < 0.01, ***p < 0.001 vs. defect; ###p < 0.001 vs. GH hydrogel. Reprinted and adapted with permission from Yoon et al., 2016 [158].