Fig. 2: Mechanisms of Immune Resistance in Pancreatic Cancer.
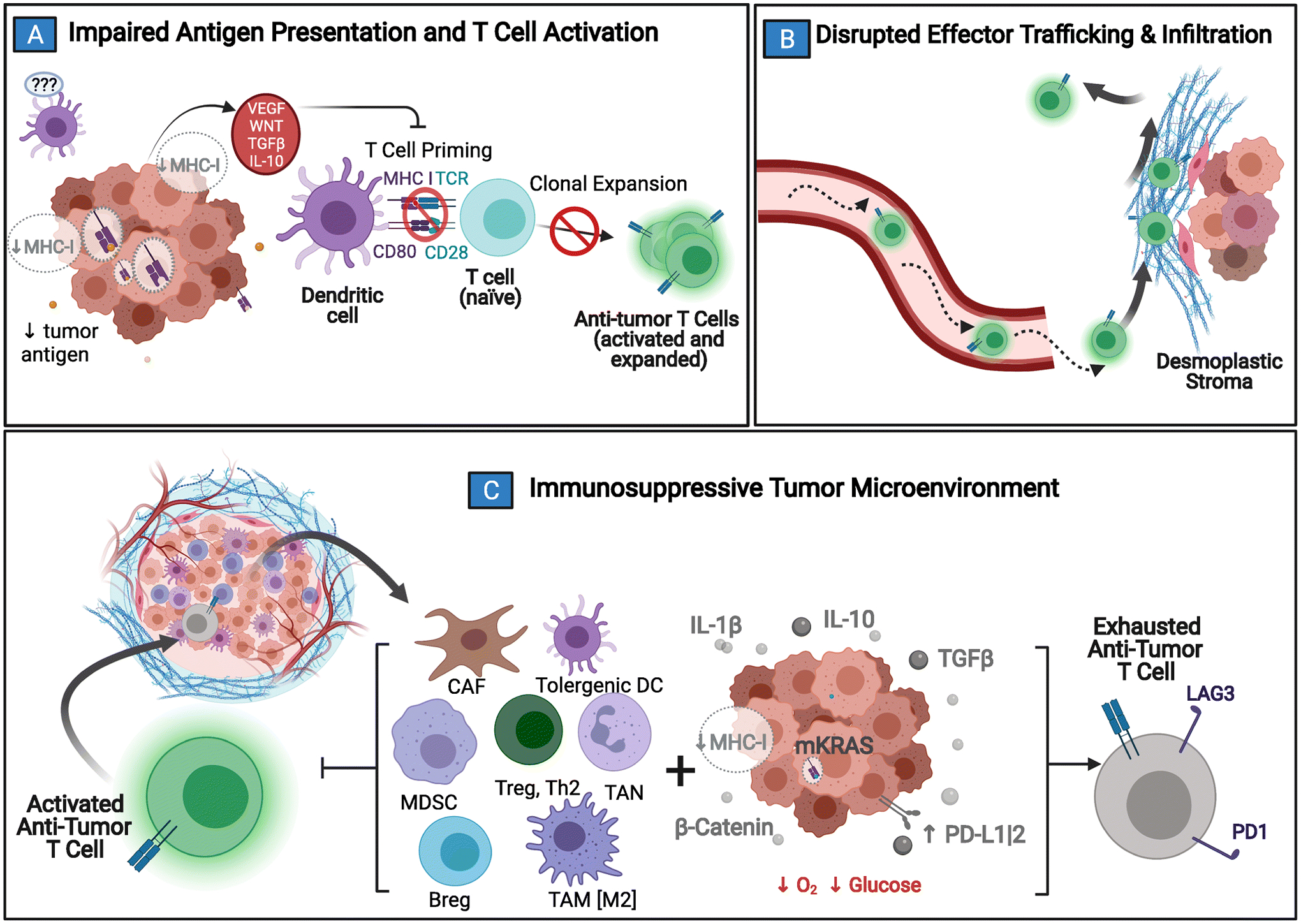
Pancreatic cancer (PDAC) has several protections against the generation and execution of an anti-tumor host-immune response. A) PDAC’s low antigen burden, sequestration of MHC/HLA-I molecules, and local tolerogenic TME signaling all contribute to poor immune surveillance and stunt normal dendritic cell maturation. Without normal antigen processing and presentation, anti-tumor effectors are not activated. B) If activation and clonal expansion of effector T cells still does manage to occur, trafficking to the tumor bed is complicated by disrupted chemokines gradients, abnormal vasculature, and the desmoplastic tumor stroma that acts as both a physical & chemical barrier to entry. C) Anti-tumor immune cells that are able to penetrate the TME are quickly exhausted by local metabolic conditions combined with immunosuppressive molecular crosstalk (e.g. upregulated interleukin 1 beta [IL-1β], transforming growth factor beta [TGFβ], interleukin 10 [IL-10], and beta-catenin [β-catenin]) between tumor and tumor-coopted immune cell populations (cancer-associated fibroblasts [CAF]; tumor associated macrophages [TAM]; tumor associated neutrophils [TAN]; tolerogenic dendritic cells [DC]; myeloid derived suppressor cells [MDSC]; type II T helper cells [Th2]; T regulatory cells [Treg]; B regulatory cells [Breg]). Adapted from “Challenges for CAR T-Cell Immunotherapy in Solid Tumors” and “Tumor Extracellular Matrix Reduces Therapeutic Efficiency in Solid Tumors”, by BioRender.com (2021). Retrieved from https://app.biorender.com/biorender-templates.
