FIGURE 2.
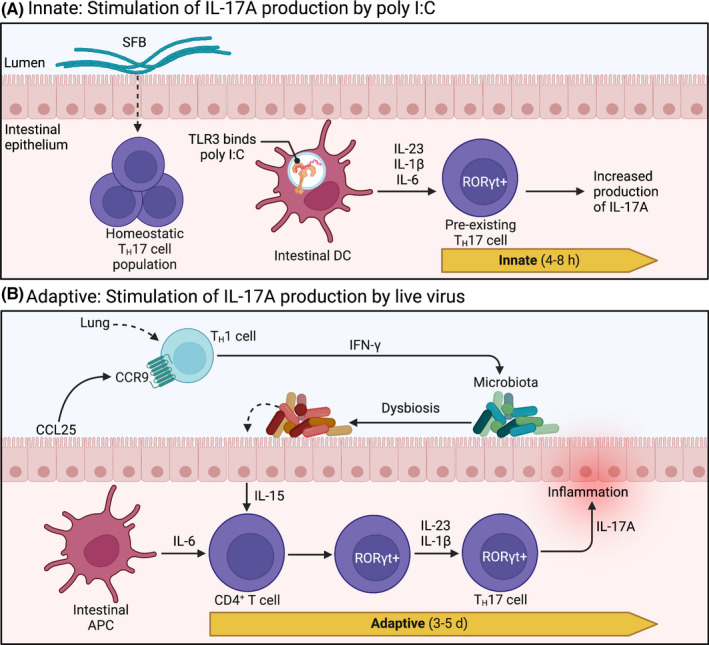
Intestinal TH17 cells in poly I:C and influenza models. (A) RORγt+ TH17 cells are constitutively expressed in the intestine and rely on commensal segmented filamentous bacteria (SFB) for homeostatic regulation. Upon intraperitoneal administration of poly I:C, intestinal dendritic cells (DC) detect poly I:C directly through TLR3, initiating production of IL‐1β, IL‐6, and IL‐23. These secreted cytokines stimulate pre‐existing resident TH17 cells to produce IL‐17A. This innate immune signaling pattern is initiated quickly, within 4‐8 h, resulting in a measurable accumulation of IL‐17A in maternal circulation at 48 h. (B) During respiratory influenza A virus (IAV) infection, CCR9+CD4+ TH1 cells—stimulated in the lungs—are recruited to the uninfected intestine by chemokine CCL25, where they produce copious amounts of IFN‐γ. Subsequent disruption of endogenous gut microbes (ie, dysbiosis) stimulates intestinal epithelial cells to produce IL‐15. In conjunction with stimulation by intestinal antigen presenting cells (APC), cytokines IL‐15, IL‐6, IL‐23, and IL‐1β prompt naive CD4+ T cells to express transcription factor RORγt, promoting polarization towards TH17 lineage. Polarized effector TH17 cells then produce IL‐17A, which has been linked to intestinal injury during IAV infection. This adaptive immune response takes up to 5 d, resulting in measurable increases in intestinal TH17 cells within 6‐7 d after IAV infection. d, days; h, hours; RORγt, retinoic acid receptor‐related orphan receptor γt; TLR, toll‐like receptor. Images were adapted from Cua and Tato (2010), 110 and the description of the common mucosal immune response to IAV was informed by Wang et al (2014). 109
