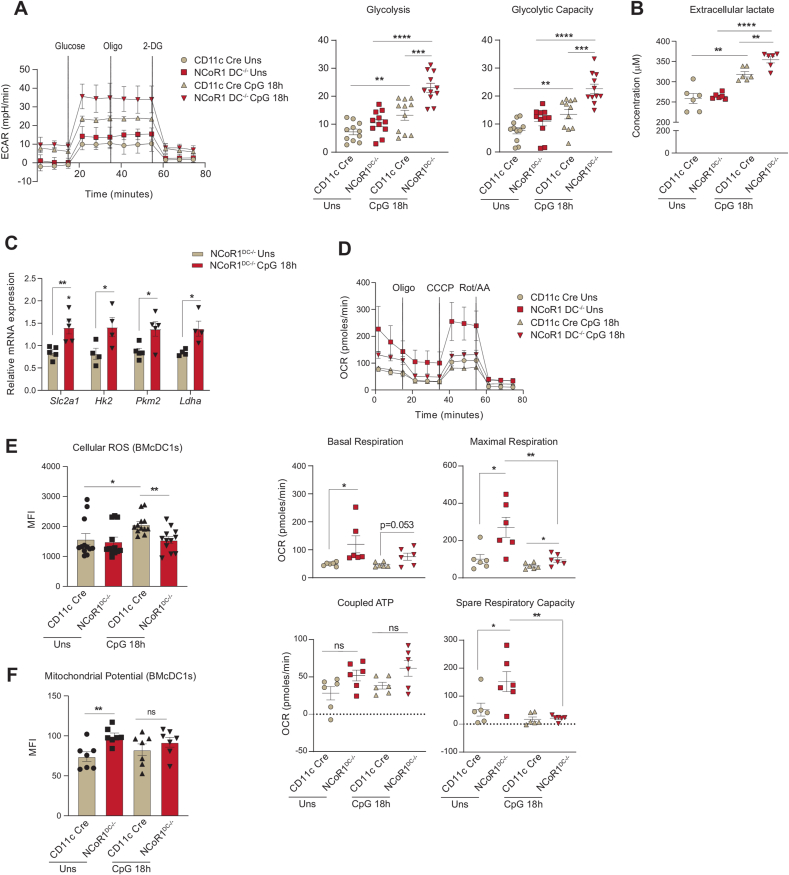
Fig. 2.
FLT3L differentiated primary BMDCs from NCoR1DC−/− mice showed enhanced glycolysis and OXPHOS as observed in mutu-cDC1 line
A. Representative line graph and scatter plots showing the glyco-stress parameters (glycolysis and glycolytic capacity) in unstimulated and 18h CpG activated control CD11c-Cre and NCoR1 ablated FLT3 differentiated BMDCs on day 9, measured using Seahorse extracellular flux analyzer. (n = 11)
B. Scatter plot depicting the levels of extracellular lactate accumulated in the culture supernatants of control CD11c-Cre and NCoR1DC−/− BMDCs before and after 18h CpG activation, at a dilution of 1:5. (n = 6)
C. Bar graphs showing the relative transcript expression of glucose transporter Slc2a1, and glycolytic genes, Hk2, Pkm2 and Ldha in unstimulated and 18h CpG activated CD11c-Cre and NCoR1DC−/− BMDCs. (n = 4–5)
D. Representative line graph and scatter plots showing the mito-stress parameters (basal respiration, maximal respiration, coupled ATP and spare respiratory capacity) in unstimulated and 18h CpG activated control CD11c-Cre and NCoR1DC−/− BMDCs on day 9 as measured using Seahorse extracellular flux analyzer. (n = 6)
E. Bar graphs with dots depicting the MFI levels of cellular ROS in unstimulated and 18h CpG activated CD11c-Cre control and NCoR1DC−/− BMcDC1s on day 9 as measured in F4/80−CD11c+CD24+ gated cells using flow cytometry. (n = 12)
F. Bar graphs with dots showing the MFI levels of mitochondrial potential upon TMRM staining in CD11c-Cre control and NCoR1DC−/− BMcDC1s on day 9 before and after 18h CpG stimulation as measured in F4/80−CD11c+CD24+ gated cells using flow cytometry. (n = 7)
*p ≤ 0.05, **p ≤ 0.01, ***p ≤0.001 and ****p ≤0.0001. p-value has been calculated using two tailed unpaired student’s t-test. Data shown in figure is combined from 4 independent experiments [A] and from 3 independent experiments [D]. Error bars represent SEM.