Figure 1.
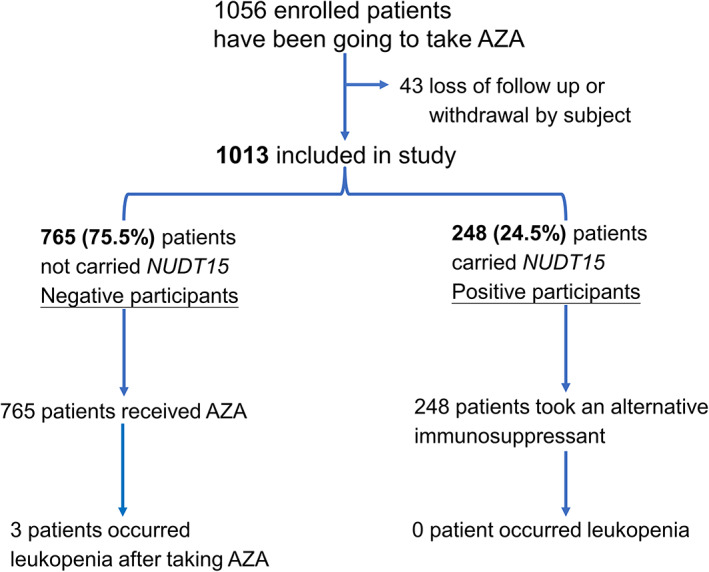
The enrollment and outcomes of prospectively genetic NUDT15 R139C testing. Azathioprine (AZA) was prescribed and provided for all participants at the time of the screening visit, but they were asked to defer taking AZA until the genetic results were available. Participants were monitored symptoms and followed for at least 6 months, with weekly or every 2‐week interviews.
