FIGURE 2.
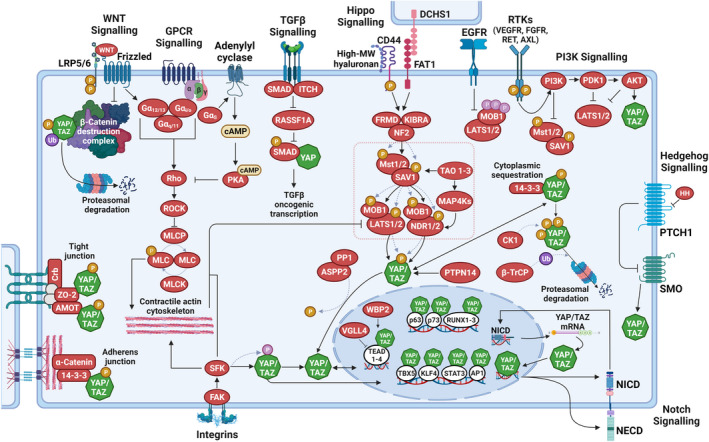
Key signals regulating YAP/TAZ activity. The transcription co‐regulators Yes‐associated protein (YAP) and transcriptional co‐activator with PDZ‐binding motif (TAZ), are predominantly regulated by phosphorylation (serine phosphorylation, orange; tyrosine phosphorylation, pink). Serine‐phosphorylated YAP/TAZ are exported from the nucleus and are either degraded in the cytoplasm via the proteasome or sequestered in the cytoplasm via 14‐3‐3 proteins or at tight‐ and adherens junctions. In their non‐serine‐phosphorylated and tyrosine‐phosphorylated states, YAP/TAZ accumulate in the nucleus, where they bind to various transcription factors, most notably those of the TEA domain (TEAD) family, to control target gene expression. In the nucleus, vestigial‐like family member 4 (VGLL4) competes with YAP/TAZ in binding to TEADs, while WW domain binding protein‐2 (WBP2) enhances the co‐activator functions of YAP/TAZ. The core of the Hippo pathway (dotted pink box) is defined by a kinase cascade composed of MST1 and MST2 kinases, large tumour suppressor (LATS)1 and LATS2 kinases and their co‐factors SAV1 and MOB1A and MOB1B. Membrane‐associated signalling events causing Hippo pathway activation include high‐molecular‐weight hyaluronan‐mediated clustering of CD44 and cell–cell signalling via Dachsous cadherin‐related 1 (DCHS1)/FAT1. Hippo pathway activation involves the phosphorylation of the core Hippo kinases, MST1/2 and LATS1/2: MST1/2 are autophosphorylated and subsequently phosphorylate LATS1/2. MST1/2 are also activated by TAO kinases. Activation of LATS1/2 causes the serine‐phosphorylation of YAP and TAZ and inhibits their transcription co‐regulator functions. PP1, together with apoptosis‐stimulating protein of p53 2 (ASPP2), antagonizes Hippo pathway activity by de‐phosphorylating YAP/TAZ. In addition to MST1/2, various upstream effectors of the LATS1/2 have been identified, including the MAP4K and TAOK families of kinases, which phosphorylate and activate LATS1/2. Nuclear Dbf2‐related (NDR)1/2 kinases act in parallel to LATS1/2 in the Hippo pathway to inactivate YAP/TAZ. The activities of the core Hippo pathway components are regulated by several upstream mechanisms. These involve various scaffolding proteins such as angiomotin (AMOT), neurofibromin 2 (NF2; also known as Merlin), kidney and brain protein (KIBRA; also known as WWC1), the protocadherin FAT1 and zonula occludens (ZO) proteins at tight junctions. Cell polarity and adhesion regulators promote LATS1/2‐mediated regulation of YAP/TAZ by altering actin dynamics and by facilitating Hippo pathway effector association. G protein‐coupled receptors (GPCR) signalling, mechanical cues and signals transduced by the extracellular matrix and matrix‐binding integrins (through FAK and SRC family kinases (SFKs)) can inactivate LATS1/2 by promoting a contractile F‐actin‐myosin cytoskeleton. SFKs also directly regulate YAP/TAZ nuclear abundance, predominantly by controlling their nuclear export rate. Soluble growth factors bind to and activate receptor tyrosine kinases (RTKs) and inactivate the Hippo pathway by stimulating PI3K–PDK1 signalling. EGFR activation causes inhibitory tyrosine phosphorylation of MOB1A/B. RASSF1A is recruited to the activated TGF‐b receptor I and subsequently targeted for degradation by the co‐recruited E3ubiquitin ligase ITCH. RASSF1A degradation then permits YAP association with SMADs and subsequent nuclear translocation of receptor‐activated SMAD2. YAP/TAZ are also regulated by WNT signalling: such as β‐catenin, YAP/TAZ also incorporates into the destruction complex and are targeted for proteasomal degradation. Upon WNT stimulation, inactivation of the destruction complex then drives β‐catenin as well as YAP/TAZ nuclear translocation. YAP/TAZ also interact with the Notch pathway: in the nucleus, YAP/TAZ can induce the gene expression of Notch receptors and/or Notch ligands to regulate Notch signalling, while the transcriptionally active Notch intracellular domain (NICD) can activate YAP1 gene transcription. Activated HH signalling leads to increased nuclear YAP abundance. AKT, Ak strain transforming; AP, activator protein; APC, adenomatous polyposis coli; cAMP, 3′ 5′‐cyclic adenosine monophosphate; CK1, casein kinase 1δ/1ε; Crb, crumbs; DVL, dishevelled segment polarity protein; EGFR, epidermal growth factor receptor; FAK, focal adhesion kinase; FGFR, fibroblast growth factor receptor; FRMD, FERM and PDZ domain containing; GSK, glycogen synthase kinase; HH, hedgehog; KLF, Krüppel‐like factor; LRP, LDL receptor‐related protein; MAP4K, mitogen‐activated protein kinase kinase kinase kinase; MLC, myosin light; MLCK, myosin light chain kinase; MLCP, myosin light chain phosphatase; NECD, Notch extracellular domain; P, phosphorylation; PDK, pyruvate dehydrogenase kinase; PI3K, phosphoinositide 3‐kinase; PKA, protein kinase A; PP1, protein phosphatase 1; PTPN, protein tyrosine phosphatase non‐receptor type; RASSF, RAS association domain family; ROCK, Rho‐associated kinase; RUNX, Runt‐related transcription factor; SFK, SRC‐family kinase; SMAD, suppressor of mothers against decapentaplegic; SMO, smoothened; STAT, signal transducer and activators of transcription; TAO, thousand and one; TBX, T‐box transcription factor; TGF, transforming growth factor; Ub, ubiquitylation; VEGFR, vascular endothelial growth factor receptor. Dotted lines indicate post‐translational modification events. Figure graphics were created with BioRender.com.
