Abstract
dear editor, Cutaneous squamous cell carcinoma (cSCC) has a cure rate of 95%. However, due to its high incidence, it is associated with more disability‐adjusted life‐years than melanoma. 1 The primary treatment of cSCC is surgical excision, with the most common approach being standard excision, where the tumour is removed with a predetermined margin of healthy tissue. Alternative approaches, such as Mohs micrographic surgery (MMS), have been proposed to improve surgical and cosmetic outcomes. In MMS, the complete resection margin is assessed intraoperatively through frozen sampling. Up to 100% margin control and lower recurrence rates have been reported with MMS, but it is resource intensive and time consuming. 2 The need for real‐time information has led to increasing interest in optical imaging techniques that enable intraoperative tumour visualization, possibly supporting surgical decision making. For example, fluorescence molecular imaging (FMI), a novel imaging method that uses tumour‐specific tracers to highlight tumour tissue, has shown potential for intraoperative margin assessment. 3 , 4 A common target for FMI is epidermal growth factor receptor (EGFR), a transmembrane receptor overexpressed in up to 90% of cSSCs. 5
In this proof‐of‐concept study, we explored the potential of FMI using cetuximab‐800CW for discrimination between cSCC and adjacent tissue. Ten patients were included with histology‐confirmed cSCC, scheduled for conventional excision or MMS. Two to three days before surgery, patients were intravenously administered cetuximab 75 mg, followed by 15 mg of cetuximab‐800CW one hour later. 3 FMI was performed intraoperatively for in vivo tumour visualization and ex vivo specimen‐driven margin assessment. 6 Surgical specimens were processed according to standard of care; additional EGFR immunohistochemistry was performed on all tissue slices. FMI was performed on tissue slices to cross‐correlate the fluorescence signal with the final histopathology. Mean fluorescence intensities of the tumour and the background were calculated to determine a tumour‐to‐background ratio (TBR). 3
The study protocol was approved by the institutional review board (METc 2019/183) and the Dutch competent authority.
In total, 12 lesions were identified, of which six were treated with MMS and six with conventional excision. One lesion was unexpectedly diagnosed as basal cell carcinoma, and for three other lesions the diagnosis of keratoacanthoma was suggested on final histology. The two conventional excisions containing cSCC showed TBRs of 2·86 and 2·35. The MMS specimens showed a mean TBR of 2·24 (range 1·82–2·62). The mean TBR of the tumour vs. adjacent tissue in the deep margin (mostly fat) was 3·07 (range 1·85–4·39). Excised cSCC specimens were analysed on the back table, including two conventional excisions and six cases of MMS. In one conventional excision, in a patient with a high‐risk cSCC that was previously irradically excised, we observed a fluorescent lesion at the deep resection margin, corresponding to a tumour‐positive margin on final histopathology (Figure 1a). The other conventional excision did not show a fluorescent signal, and the minimal deep margin was 4·8 mm.
Figure 1.
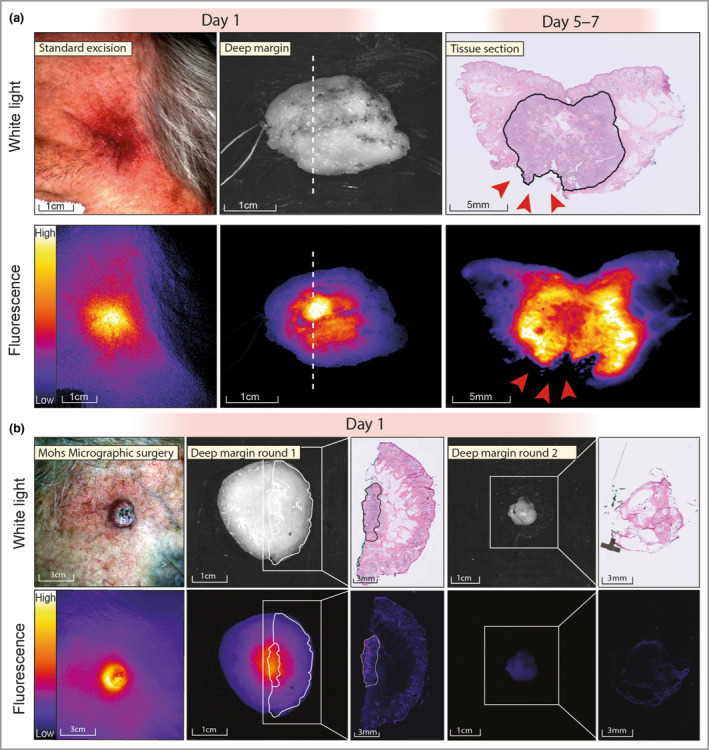
Fluorescence molecular imaging during standard excision and Mohs micrographic surgery (MMS). (a) Fluorescence molecular imaging before standard excision of a temporal, subdermal tumour that was earlier irradically removed. Ex vivo imaging of the specimen showed a fluorescent lesion at the deep resection margin, correlating with a tumour‐positive margin on histopathology (red arrowheads). (b) Fluorescence molecular imaging during an MMS procedure. In vivo imaging shows a sharply demarcated fluorescent lesion. Ex vivo imaging shows a fluorescent lesion at the deep resection margin, which colocalizes with tumour on haematoxylin and eosin histopathology. The second MMS round did not show any remaining fluorescence signal, and no tumour was found on histopathology.
In five of six first‐stage MMS specimens, we observed a fluorescent lesion at the deep resection margin (Figure 1b). In these five cases, three tumour‐positive margins were identified. In the other two cases, the margin was not tumour‐positive, but the fluorescent signal colocalized with tumour tissue on additionally obtained tissue sections located closer to the skin. As such, the false‐positive signal resulted from limited depth information of the fluorescence signal, leading to detection of tumours localized under the surface. No tumour was present on histopathological examination in the MMS specimen that did not show a fluorescent lesion. The three patients with MMS with a tumour‐positive margin required an additional excision (i.e. after stage 1). None of these showed a fluorescence signal, and all were tumour‐negative on final histopathology. We determined the overall performance of FMI for margin assessment using all conventional excisions and all MMS excisions. We obtained 100% sensitivity, 63% specificity, 100% negative predictive value and 57% positive predictive value. The overall accuracy was 75%.
In the basal cell carcinoma, we found a TBR of 2·22. The three keratoacanthomas showed TBRs of 0·77, 1·66 and 1·69. One patient with two keratoacanthomas had a long history of immunosuppression use and showed substantial actinic damage. This resulted in high fluorescence in the background, and the tumours did not show increased fluorescence signal compared with this background signal during in vivo imaging. EGFR immunohistochemistry showed weak to strong expression in all cSCCs, colocalizing with the fluorescence signal. Low EGFR expression was found in the basal cell carcinoma. The keratoacanthomas showed no EGFR expression, as reported previously. 7
This proof‐of‐concept study demonstrates that FMI using the fluorescent tracer cetuximab‐800CW can differentiate between tumour and adjacent tissue with high contrast. FMI detected all tumour‐positive margins intraoperatively within seconds. FMI could be valuable for patients with a large or complex cSCC, where obtaining 100% intraoperative margin control is anticipated to be critical. However, it seems less useful for keratoacanthoma‐like cSCC or in patients with excessive actinic damage. Future studies should determine the clinical value of FMI in surgery of high‐risk cSCCs, ideally with new imaging methodologies that deliver improved depth information. 8
Author contributions
Jasper Vonk: Conceptualization (equal); formal analysis (lead); investigation (lead); methodology (lead); writing – original draft (lead). Jaron Gérard de Wit: Conceptualization (equal); formal analysis (lead); investigation (lead); methodology (lead); writing – original draft (lead). Floris Jan Voskuil: Conceptualization (equal); funding acquisition (equal); investigation (equal); methodology (equal); supervision (equal); validation (equal); writing – review and editing (equal). Marjolein Koldijk: Methodology (equal); supervision (equal); writing – review and editing (equal). Emoke Racz: Supervision (equal); writing – review and editing (equal). Wouter T.R. Hooghiemstra: Methodology (equal); resources (equal); writing – review and editing (equal). Jan Johannes Doff: Methodology (equal); writing – review and editing (equal). Gilles Diercks: Supervision (equal); writing – review and editing (equal). Gooitzen M. van Dam: Conceptualization (equal); methodology (equal); writing – review and editing (equal). Max Johannes Hendricus Witjes: Conceptualization (equal); funding acquisition (equal); methodology (equal); project administration (equal); writing – review and editing (equal). Sebastiaan A.H.J. de Visscher: Conceptualization (equal); investigation (equal); methodology (equal); supervision (equal); writing – review and editing (equal).
Acknowledgments
We thank all patients who participated in this study. We thank Marloes van Kester for her help in designing the study, and Alet Leus and Aniek Lamberts for their help in recruiting patients. We thank the lab technicians from the Department of Pathology for their help in tissue processing.
Funding sources: the study was funded by ‘Stichting BOAA’ of the Dutch Society of Oral and Maxillofacial Surgery.
Conflicts of interest: G.M.vD. is a member of the scientific board of SurgVision BV, and founder, shareholder and CEO of TRACER Europe BV (Groningen, the Netherlands).
Data availability: All data are available upon reasonable request.
J.V. and J.G.dW. contributed equally to this work and share first authorship.
References
- 1. Urban K, Mehrmal S, Uppal P et al. The global burden of skin cancer: a longitudinal analysis from the global burden of disease study, 1990–2017. JAAD Int 2021; 2:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Lee CB, Roorda BM, Wakkee M et al. Recurrence rates of cutaneous squamous cell carcinoma of the head and neck after Mohs micrographic surgery vs. standard excision: a retrospective cohort study. Br J Dermatol 2019; 181:338–43. [DOI] [PubMed] [Google Scholar]
- 3. Voskuil FJ, de Jongh SJ, Hooghiemstra WTR et al. Fluorescence‐guided imaging for resection margin evaluation in head and neck cancer patients using cetuximab‐800CW: a quantitative dose‐escalation study. Theranostics 2020; 10:3994–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Keulen S, Nishio N, Fakurnejad S et al. The clinical application of fluorescence‐guided surgery in head and neck cancer. J Nucl Med 2019; 60:758–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mulvaney PM, Massey PR, Yu KK et al. Differential molecular expression patterns associated with metastasis in cutaneous squamous cell carcinoma: a systematic review and meta‐analysis. J Invest Dermatol 2021; 141:2161–9. [DOI] [PubMed] [Google Scholar]
- 6. Voskuil FJ, Vonk J, van der Vegt B et al. Intraoperative imaging in pathology‐assisted surgery. Nat Biomed Eng 2022; 6:503–14. [DOI] [PubMed] [Google Scholar]
- 7. Koller M, Qiu SQ, Linssen MD et al. Implementation and benchmarking of a novel analytical framework to clinically evaluate tumor‐specific fluorescent tracers. Nat Commun 2018; 9:3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Torres VC, Li C, Brankov JG, Tichauer KM. Model‐based system matrix for iterative reconstruction in sub‐diffuse angular‐domain fluorescence optical projection tomography. Biomed Opt Express 2021; 12:1248–62. [DOI] [PMC free article] [PubMed] [Google Scholar]


