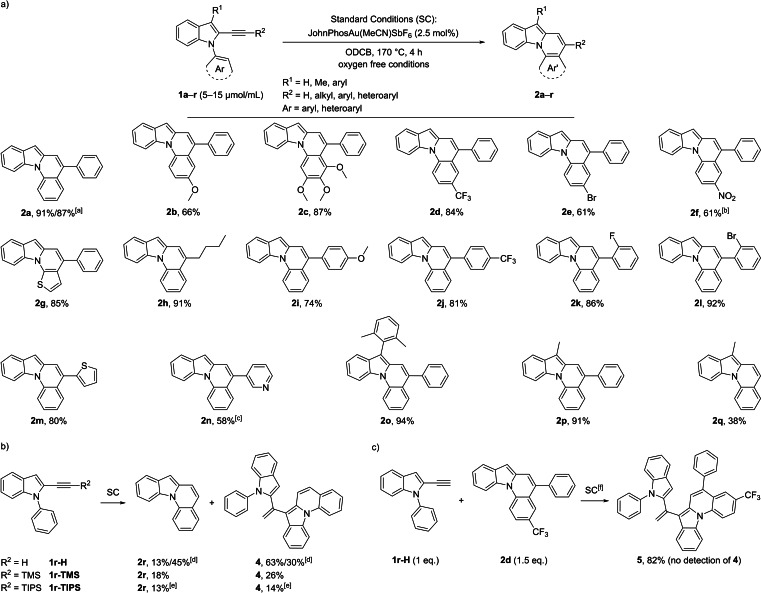
Scheme 4.
a) Gold‐catalyzed cycloisomerization of 1 forming indolo[1,2‐a]quinoline derivatives 2. b) Occurrence of dimeric side‐product 4 for derivatives of 1 r. c) Trapping experiment to explain the abundance of 4. [a] 150 μmol scale: 91 % yield; 3.48 mmol scale: 87 % yield. [b] SC for 4 h, then,+2.5 mol % catalyst, 180 °C, 12 h. [c] SC for 4 h, then,+7.5 mol % catalyst, 180 °C, 16 h, then,+10 mol % catalyst, 180 °C, 24 h. [d] Using SC with c(1 r‐H)=15.3 μmol/mL: yield (2 r)=13 %, yield (4)=63 %; with c(1 r‐H)=2.51 μmol/mL: yield (2 r)=45 %, yield (4)=30 %. [e] 20 mol % of catalyst was used and the mixture was stirred at 180 °C for 16 h; 50 % of the starting material 1 r‐TIPS was recovered. [f] 150 °C.