FIGURE 1.
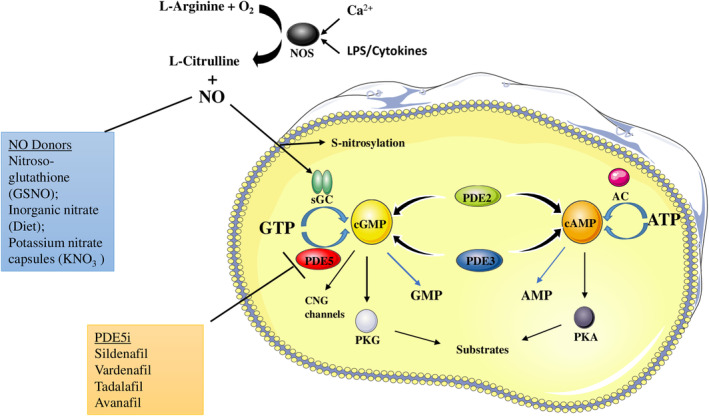
Nitric oxide (NO)/cyclic guanosine monophosphate (cGMP)/protein kinase G (PKG) transduction signaling pathway in platelets. NO is produced by oxidation of L‐arginine by the NO synthase (NOS) enzymes, and acts as an activator for soluble guanylyl cyclase (sGC), which induces cGMP production through guanosine‐5′‐triphosphate (GTP) phosphorylation. Many NO donors, endogenous and exogenous ones, augment NO bioavailability, thus leading to a major activation of the cGMP pathway. cGMP can both inhibit and enhance cyclic adenosine monophosphate (cAMP) production in platelets, by allosterically inhibiting phosphodiesterase 3 (PDE3)—a cAMP inhibitor, and by stimulating phosphodiesterase 2 (PDE2) that degrades cAMP, so NO/cGMP can inhibit platelet activation either in a cGMP‐dependent PKG–dependent (NO‐cGMP‐PKG) or –independent (NO‐cGMP‐PDE3A‐cAMP‐PKA) pathway. cGMP binds to the three different effector proteins, PKGs, PDEs, and the cyclic nucleotide‐gated cation channels (CNG channels) that mediate sensory transduction in cells. Phosphodiesterase 5 (PDE5) is a cGMP‐specific phosphodiesterase that targets it and inhibits the crucial NO/cGMP/PKG signaling pathway. In presence of PDE5 inhibitors (PDE5i), PDE5 cannot exert its hydrolyzing function, by allowing cGMP to continue its platelet inhibitory function through PKG. AMP, adenosine monophosphate; ATP, adenosine triphosphate; PS, lipopolysaccharide; PKA, protein kinase A.
