Figure 1.
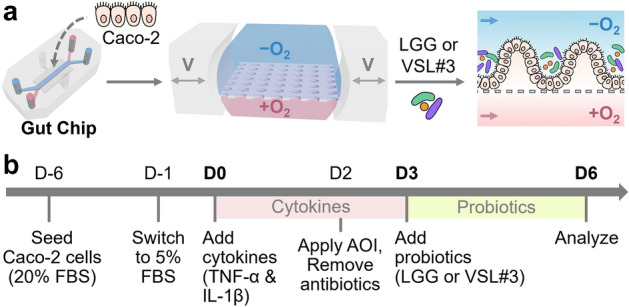
The experimental design of the creation of a Leaky Gut Chip for validating probiotic effects. (a) The workflow illustrating the growth of intestinal epithelial Caco-2 cells in a Gut Chip that employs a physiological oxygen gradient and host-probiotics co-cultures with either LGG or VSL#3 cells. The “−O2” and “+O2” mean the absence and the presence of oxygen in the culture medium, respectively. Bidirectional grey arrows in the middle schematic show cyclic mechanical deformations in a Gut Chip. V, cyclic vacuum suctions. A dashed line in the right schematic shows the location of a porous basement membrane in a Gut Chip. Blue and pink arrows display the direction of the culture medium in a Gut Chip. (b) An experimental timeline to induce a leaky epithelial barrier and cellular inflammation followed by probiotic amelioration. Epithelial Caco-2 cells are cultured in a Gut Chip under flow and motions for 5–6 days at a high serum condition (20% (v/v) FBS), then switched to a low-serum (5% FBS) culture medium that contains a cytokine cocktail (TNF-α and IL-1β) for 3 days. Probiotic co-cultures were performed after epithelial cells were stabilized to an oxygen gradient and an antibiotic-free condition. During the probiotic co-cultures, cytokine treatment was discontinued. AOI, anoxic–oxic interface; Abx, antibiotics. “D” indicates the day of cultures. We set D0 as the first day of cytokine treatment. D-1 and D-6 denote the pre-culture of Caco-2 cells in a Gut Chip on 1 and 6 days before the cytokine treatment, respectively. The pink and green boxes indicate the period of a cytokine challenge and a probiotic co-culture, respectively.
