Fig. 5.
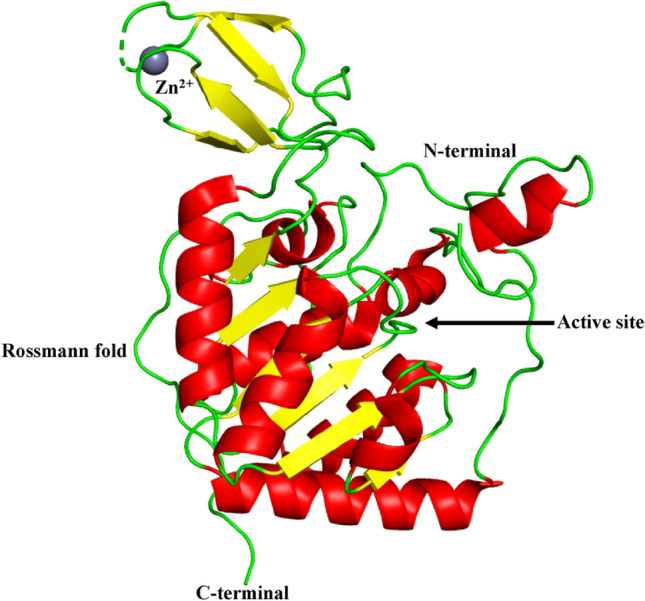
Structure of SIRT6 (PDB ID: 3K35). SIRT6 has two globular domains, each with eight α-helices and nine β-strands. A large Rossmann fold domain for NAD + binding is made up of six-stranded (β1, β2, β3, β7, β8, and β9) parallel β sheet amid between two helices (α6 and α7) on one side and four helices (α1, α4, α5, and α8) on the other side. The smaller domain, which contains a zinc-binding motif is formed by two extending loops (linking β 3 and α6) from the large domain and includes a three-stranded antiparallel β -sheet (β 4, β 5, and β 6). A short loop replaces the helix bundle, interacting with the loop between α2 and α3, engaging with a small area on the zinc binding unit. The dashed line represents the missing residues along the protein backbone
