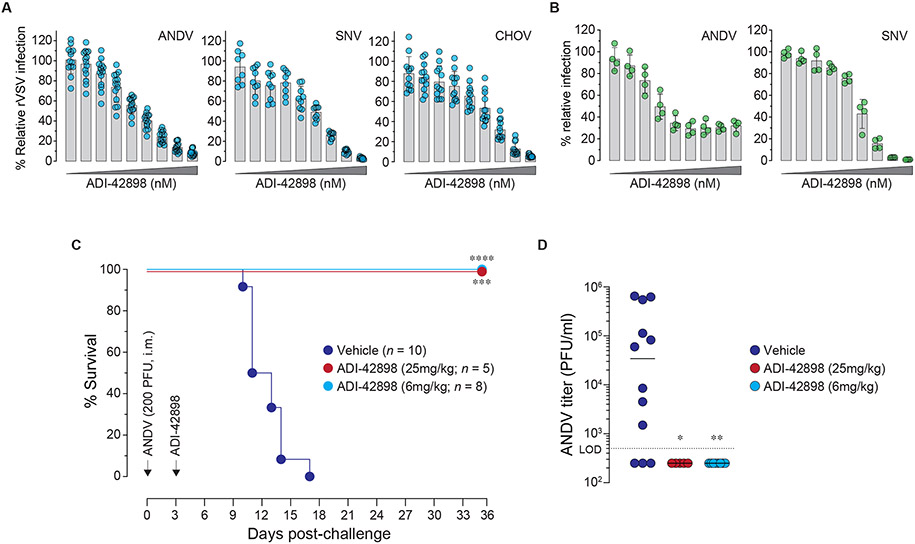
Fig. 8: Broad pan-hantavirus neutralization and protection by ADI-42898.
(A) Potency of ADI-42898 to block infection of Vero cells by rVSVs bearing Gn/Gc from the indicated NWH. Viruses were exposed to a 3-fold mAb dilution series starting at 100 nM. Averages ± s.d, n=14 from five experiments (ANDV); n=9 from three experiments (SNV); n=12 from four experiments (CHOV). (B) Potency of ADI-42898 to block ANDV (strain Chile-9717869) and SNV (strain CC107) infection of HUVECs with a 3-fold mAb dilution series starting at 300 nM. Averages ± s.d, n=4 from two experiments. (C) Syrian golden hamsters were challenged with ANDV (200 PFU, i.m.; strain Chile-9717869) followed by treatment with a single dose of ADI-42898 (~25 mg/kg or ~6 mg/kg, i.p.) at 3 days post-virus exposure. Mortality of hamsters was monitored for 35 days. Averages from two experiments, n=5 (~25 mg/kg dose); n=8 (~6 mg/kg dose), n=10 (vehicle). Untreated versus mAb-treated animals, Mantel-Cox test: ***, P=0.0003; ****, P<0.0001. (D) Serum virus titers of Syrian golden hamsters on day 8 post ANDV challenge were determined by plaque assay. Untreated versus ADI-42898-treated animals, un-paired Kruskal-Wallis test: *, P=0.03; **, P=0.002. L.O.D., limit of detection.