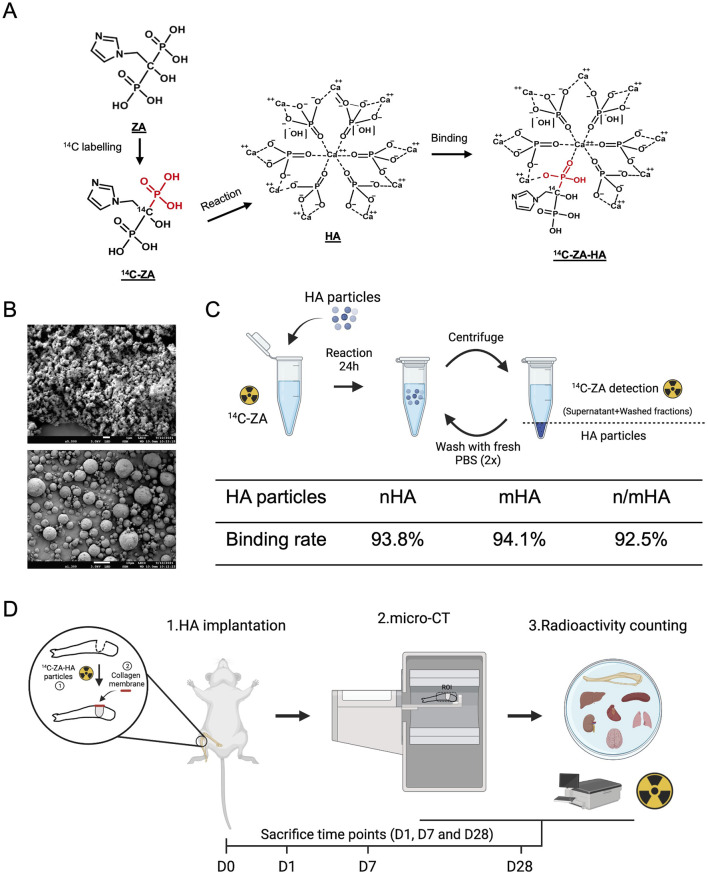
FIGURE 1.
Hydroxyapatite particle characterization, labelling and the study design. (A) Shows the speculated mechanism of 14C-ZA and HA binding, which makes the evaluation of in vivo HA biodistribution possible. (B) Shows the shape and size distribution of nano- and micro-sized HA particles used in this study. (C) Schematic showing the process of 14C labelling for nHA, mHA and n/mHA and its binding rate. Panel C was made on BioRender.com. (D) Shows a schematic diagram of the timeline and the evaluation techniques used in the evaluation of in vivo HA particles biodistribution. Briefly, the HA particles (nHA, mHA and n/mHA) were fabricated into uniform pellets, which were implanted in the tibia defect and covered by a collagen membrane. At each time point (D1, D7 and D28), the tibia was scanned by micro-CT to detect the local distribution of implanted HA particles and collected samples (proximal tibia and vital organs) were further analyzed for the detection of radioactivity which indirectly indicated the biodistribution of 14C-ZA labelled HA particles. This figure was made on BioRender.com.