Table 1.
MRI markers of CSVD.
| Marker | Definition | Pathophysiology | Example |
|---|---|---|---|
| Cerebral microbleed | Small, rounded areas (diameter ⩽ 10 mm) of signal void. Best seen on SWI or T2*weighted GRE as decreased signal and blooming effect. | Hemosiderin-laden macrophages at the site of previous extravasation of blood from small cerebral arteries. Other mechanisms might include microaneurysms and hemorrhagic transformation of microinfarcts. |
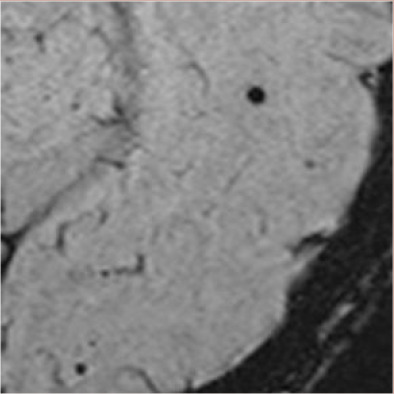
|
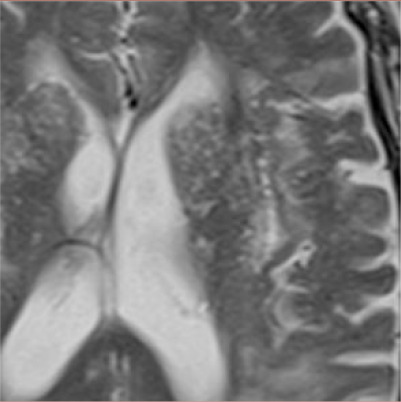
| White matter hyperintensity | Increased signal on T2-weighted and FLAIR MRI. Mainly located in white matter; subcortical hyperintensities in deep gray matter and brainstem are not included in WMHs. | Gliosis, demyelination, blood brain barrier impairment. Associated with abnormalities of small cerebral arteries. |
|
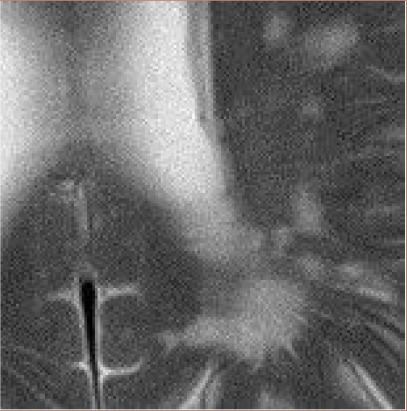
| Lacune | Round or ovoid fluid filled cavity (3-15 mm diameter in the axial plane) mostly in subcortical regions; hyperintensity on T2-weighted MRI; decreased signal in FLAIR (often with hyperintense halo) and T1-weighted images. Signal characteristics similar to CSF. | Usually due to infarction in the territory of a single perforating artery, but can also result from small hemorrhages or occlusion of a larger artery (e.g., striatocapsular infarct). |
|
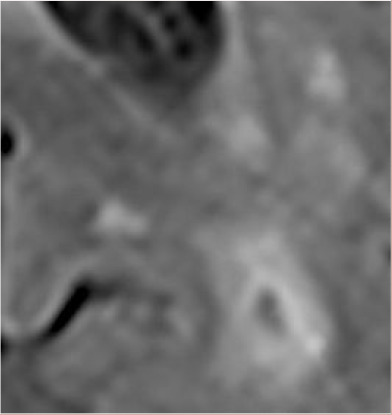
| Enlarged perivascular spaces | Fluid-filled spaces that follow the typical course of a vessel through gray or white matter. Found in the basal ganglia or centrum semiovale. Similar signal intensity with CSF; increased T2-weighted signal decreased signal on FLAIR and T1-weighted MRI. | Enlargement of the anatomical compartment between vessel walls and the glia limitans of the blood–brain barrier; may reflect impaired interstitial fluid drainage from the brain associated with structural and functional consequences of CSVD. When located in the basal ganglia, EPVS are associated with arteriolosclerosis. EPVS in the centrum semiovale appear to be related to CAA. |
|
