Abstract
Research into crop yield and resilience has underpinned global food security, evident in yields tripling in the past 5 decades. The challenges that global agriculture now faces are not just to feed 10+ billion people within a generation, but to do so under a harsher, more variable, and less predictable climate, and in many cases with less water, more expensive inputs, and declining soil quality. The challenges of climate change are not simply to breed for a “hotter drier climate,” but to enable resilience to floods and droughts and frosts and heat waves, possibly even within a single growing season. How well we prepare for the coming decades of climate variability will depend on our ability to modify current practices, innovate with novel breeding methods, and communicate and work with farming communities to ensure viability and profitability. Here we define how future climates will impact farming systems and growing seasons, thereby identifying the traits and practices needed and including exemplars being implemented and developed. Critically, this review will also consider societal perspectives and public engagement about emerging technologies for climate resilience, with participatory approaches presented as the best approach.
We review the effects of climate change on farming systems, suggest key traits and technologies relevant to developing climate-resilient crops, and discuss the need for public engagement.
Introduction
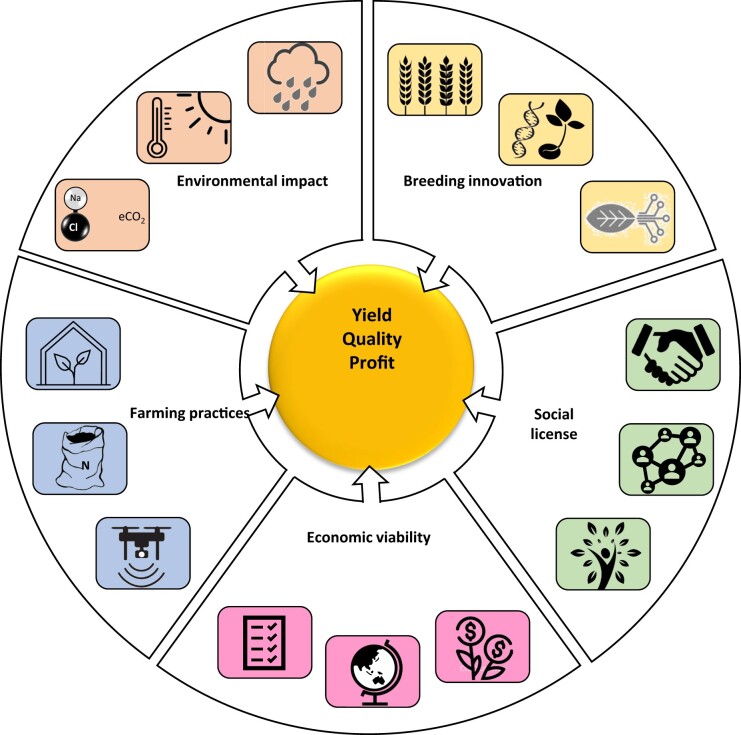
Research into crop yield and resilience has underpinned global food security, evident in the tripling of yields in the past 5 decades (Ritchie and Roser, 2021). The challenges that global agriculture now faces are not just to feed 10+ billion people within a generation, but to do so under harsher, more variable, and less predictable climates, and in many cases with less water, more expensive inputs, and declining soil quality. Therefore, the challenges presented by climate change are not simply to breed for a “hotter drier climate,” but to enable resilience to floods and droughts and frosts and heat waves, possibly even within a single growing season. The agri-tech community needs to re-envisage better crops, technologies, and cropping systems suited to more dynamic and agile farm systems to better deal with highly variable and more extreme environments. How well we prepare for the coming decades of climate variability will depend on our ability to modify current practices, innovate with novel breeding methods, and communicate and work with farming communities to ensure viability and profitability (Figure 1). In this review, we highlight how future climates will impact farming systems, and suggest key crop traits and practices relevant to addressing these challenges. We consider these innovations from a societal perspective and highlight the need for greater public engagement regarding emerging technologies for climate resilience.
Figure 1.
The considerations steering future agriculture. Created with BioRender.com.
Future climates will impact farms and alter growing seasons
The pace with which we are confronted by the realities of climate change is increasingly staggering, as is our certainty of it redefining world food production (Brás et al., 2021; He et al., 2022). The last decade alone has produced a surge in compound drought and heatwave events. He et al. (2022) suggested that >92% of the wheat-growing districts experienced at least one compound drought/heatwave event per season, with frequency and duration increasing by >28% since the 1980s. Globally, climate variability has affected food security through rainfall and temperature changes, and the increasing frequency of extreme climatic events (IPCC, 2019), compounded by likely increases or changes in the distribution of pests and diseases in many regions (Canto et al., 2009; Pangga et al., 2011; Juroszek and von Tiedemann, 2013). Climate changes have reduced broadacre farming profitability, as evidenced by a 22% reduction in Australia since 2000 (Hughes et al., 2019). To better understand the likely effects caused by climate change, we need to understand the individual risks posed, the integrative nature of abiotic stresses and responses, and the changing risk profiles on farms of more variable weather patterns and altered growing seasons that introduce more complexity.
Impact of temperature changes
How crop yields respond directly to temperature change is a complex question. Yield losses are likely when the temperature exceeds a species-specific ideal, but this may in turn increase yields in some colder climates where this optimum is not currently met (Porter and Gawith, 1999; Teixeira et al., 2013; Sanchez et al., 2014; Asseng et al., 2015; Zhao et al., 2016). Such increases are not broad in nature and will require local adaptation of crops to new climates (Teixeira et al., 2013; Rosenzweig et al., 2014; Asseng et al., 2015). Globally reduced yields of major crops due to rising temperatures were quantified by Wang et al. (2020), with >95% certainty of yield reduction in rice, maize, and soybean estimated at 5.6%, 7.1%, and 10.6% losses, respectively, for every 1°C of global warming, and 89% probability of loss in wheat of ∼2.9% °C−1. Under a 2°C global warming scenario, the net effect of temperature on major crop production was estimated at a loss ranging between 3% and 13%.
Increasing temperature variability will also lead to changes in the likelihood of frost events in some agricultural systems, both contracting and expanding in different regions. Some regions will see improved frost conditions for many crop types, for example California or the UK (Atucha-Zamkova et al., 2021; Parker et al., 2021), but risks will be increased particularly in Europe and Asia (Zohner et al., 2020). In Australia, frost damage to crops causes between AUD$120M and $700M per year (Barlow et al., 2015; Zheng et al., 2015). Southern Australia, producing the majority of the country’s cereal crop production, has seen an increase of 26 days in the frost window in the period 1960–1990, even with the warming trend currently observed (Crimp et al., 2016). In some cases, frosting is occurring on average 4 weeks later than in 1960. A recent case was the frost events of early April 2021 in Europe: 80% of France’s fruit trees, oilseed production, potatoes, sugar beets, and vineyards were damaged, and the damage was also seen in Italy, the UK, and the Czech Republic (Lamichhane, 2021).
Impact of drought
The likelihood and severity of drought increase with climate change, as does the risk to yield of major staple crops. In some geographical localities, current genetic gains in yield are not offsetting losses ascribed to climate change (Hochman et al., 2017). Future wheat production under moderate drought conditions has a 58% probability of falling below long-term averages, particularly in the USA and Canada, where such losses may approach >80% under future exceptional drought conditions (Leng and Hall, 2019). Wheat production is estimated to fall by up to 7% under a 1.5°C warming above pre-industrial conditions scenario, and up to 11% under a 2°C scenario (Liu et al., 2019; Zaveri and Lobell, 2019). Future risk at the end of the 21st century for yield losses for maize is ∼6% higher than the historical risk (especially in India), while soybeans (up to 16%) and rice (up to 19.5%) had much higher risks (Leng and Hall, 2019). Areas of South East Asia seem mostly at risk of rice yield losses, while soybean losses will predominate in the USA, Russia, and India.
Rainfall changes due to climate change are local rather than general in nature; average precipitation globally has increased ∼2 mm year−1 per decade, but this is generally restricted to the tropics while there have been decreases in other latitudes (Hartmann et al., 2013; Beck et al., 2019). Of serious concern are the increasing likelihood of extreme rainfall intensities and the changing nature of flooding events (Wasko et al., 2021). In some regions, rainfall event duration is becoming shorter, and longer dry periods are observed between events (Chang et al., 2016), but the magnitude of extreme rainfall events is increasing with less uniform rainfall patterns (Wasko and Sharma, 2015; Wasko et al., 2021). Fewer “average” flooding events will occur and instead larger, damaging floods will occur more frequently—brutally apparent in Eastern Australia as it enters a third consecutive La Niña event in 2022 (BOM, 2022). This has serious implications for both infrastructures and soil and water quality considerations.
Soil constraints from climate change
Soil salinization rates are expected to be affected by extreme climatic events (IPBES, 2018; Talukder et al., 2021). Increased drought risk in regions with shallow water tables will lead to increased surface salinity due to evapotranspiration (ET) and capillary rise in the soils. Conversely, extreme wetting events increase weathering rates, raising salt levels in rivers and streams, and subsequently in fields with repeated irrigation. Increased rainfall will also force water tables to rise, bringing salt to the surface and increasing salinity on drying. Increased need for aquifer-based irrigation water in dry years also poses a risk of increasing salinity; overdrawing of well waters in coastal areas or adjacent to saline aquifers increases the movement of water from those saline subsurface sources. This is exacerbated in coastal regions by expected ocean level rises (Corwin, 2021).
Changed inputs due to soil and plant growth changes
Weather variability around rainfall will also affect nutrient availability. For example, an elevated risk of extreme summer rain events will increase the rate of loss of nitrogen (N) and phosphorous from agricultural lands, with nitrates lost due to leaching and phosphates lost in soil particulates transferred to waterways with high rainfall events (Jaynes et al., 2001; Singer et al., 2011; Lychuk et al., 2021). Increased temperature during vegetative growth phases can result in accelerated growth rates, which in turn place further demand on soil nutrient availability (Rawson, 1988). Conversely, C3 plant species have been found to have lower protein/N content with elevated [CO2], averaging 14% lower plant tissue N concentration in experimental systems (Cotrufo et al., 1998; Jablonski et al., 2002), though conflicting evidence exists (Dier et al., 2019). As plant protein/N content is reduced, plant litter has a corresponding lower N content; when combined with reduced litter breakdown rates, this leads to lower soil N levels and an increased N fertilizer requirement (Uddling et al., 2018; Ainsworth and Long, 2021).
The soil microbiome is not impervious to climatic variation. Microbial communities, essential for efficient plant growth and soil health, are heavily impacted by drought conditions (Hueso et al., 2012; Barnard et al., 2013). Loss of these microbes results in reduced organic matter mineralization and nutrient recycling. Such losses could be crucial in legume crop performance under drought conditions, with microbe losses impacting nitrogen assimilation capacity (Marschner et al., 2004; Lau and Lennon, 2011; Prudent et al., 2020). For a review of risks to microbiome and mitigation strategies, see Dubey et al. (2019) and Jansson and Hofmockel (2020).
Current strategies used for developing stress resilience
The impacts of individual abiotic stresses are serious enough in isolation, but the increased variability in temperature and precipitation events leads to the risk of multiple—possibly contrasting or combinatorial—stress events occurring within a single growing season. There is an increasing possibility of heat and frost events occurring around anthesis in cereal crops, and/or drought and flood occurring in a single season (IPCC, 2022; Kreibich et al., 2022). This complicates efforts to overcome or prepare for such stress events.
How do we attempt to develop crops for environments we do not yet understand or cannot predict with sufficient accuracy? Breeding methods rely on measurable traits and target environments. If they are varied and are unreliable, absent, or even opposite in sign (e.g. drought versus high rainfall), then key alleles for adaptation can be lost. This is of concern if there is an economic cost of these traits or alleles in the absence of key stress (Rosielle and Hamblin, 1981; Atlin and Frey, 1989). Given the increasing variability found in many different environments, this is increasingly likely (Power et al., 2017). For example, between 2010 and 2022, South Eastern Australia experienced every decile of annual rainfall—how then does one define the “average environment” for a crop, let alone develop crops for it using conventional means?
Modern methodologies include examining line performance under diverse environmental conditions to assess genotype × environment interactions, using target population of environments (TPEs) to select sites varying in climate, soil, and resource availability (as an example, see Crespo-Herrera et al., 2021 for Indian TPEs). Utilizing this framework in successive years with variable climate at each site provides the structural underpinning to assess trait stability and importance. For a review of methodologies and applications, see Chenu et al. (2018), Cooper et al. (2021), Yan (2021), and Cooper and Messina (2022). While the selection environments used can incorporate slow and subtle changes in climate very well (Richards et al., 2014), abrupt climate change when temperature increase reaches local tipping points is likely to occur, rather than only gradual change (Alley et al., 2003; Boulton et al., 2020). For example, the mid-1970s abrupt change in Pacific Ocean circulation patterns related to the El Niño-Southern Oscillation Index (Graham, 1994) and the early twentieth-century warming that coincided with extreme climatic events (Hegerl et al., 2018).
Current efforts to develop crops with improved abiotic stress tolerance have generally repeated the efforts of the past with limited success. However, potential exists: including the use of small breeding cycles (1–3 years) for rapid selection of adaptation genes (Atlin et al., 2017); the use of evolutionary populations and mixtures, allowing for change in gene frequency through selection of the most suited materials in a field cycle from within heterogeneous populations (Ceccarelli and Grando, 2020); the inclusion of diverse stress alleles from wild populations (Dempewolf et al., 2014); and incorporation and sometimes combinations of simple morphological traits to improve resilience (Hunt et al., 2018). Snowdon et al. (2021) expressed confidence that continued per se breeding should deliver improved performance through fixation of existing genetics, modern breeding methods, and exploiting of climate adaptation through improved understanding of genotype × environment × management interactions.
Successful efforts to improve crop performance have used a simple physiological understanding of the interrelatedness of traits in directing breeding or agronomic improvement for a given environmental constraint. For example, Passioura (1977) described a simple model for yield determination in water-limited environments, describing grain yield in terms of water use or ET, water use efficiency (WUE), and harvest index (HI) throughout the growing season as:
WUE can be further partitioned into the portion of total water transpired by the crop (T/ET) and the transpiration efficiency of biomass production (W), resulting in:
The benefit of this framework approach reflects the simplicity of understanding and then directing change in the broad processes by which crops yield harvestable products in water-limited environments (Condon et al., 2004).
Contrasting with this crop-based framework approach is laboratory-based efforts focusing on narrowly defined measures of “resistance” to abiotic stress. As reviewed by Passioura (2020), translation of laboratory-based efforts to resist abiotic stress has mostly failed due to a singularity of focus on, say, a given stress in a given tissue; rather than optimizing resource use efficiency across the growing season, as encompassed in the Passioura model. Similarly, the identification of cereals with low salt accumulation over a lifetime was more successful than targeting genes, which improved resistance to a short, defined salt stress shock (Munns et al., 2012). The lack of success in validating laboratory-generated traits in field environments is highlighted by Simmons et al. (2021) and summary of maize biotech research efforts by Corteva Agriscience; only 1.3% of the traits were validated in field conditions, with the vast majority of traits leading to a reduced yield potential. In short, by failing to treat crops as integrated systems, laboratory-based improvement efforts have instead become focused on single parts of the whole, with understandably mixed results.
Opportunities for applying new genetic technologies
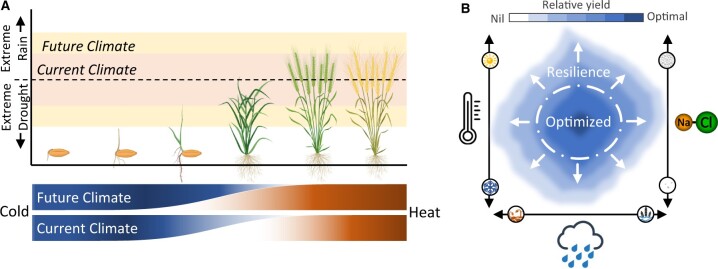
Given the uncertain nature of the likelihood and intensity of disruptions in future growing seasons both traditional breeding methods and new molecular technologies must be linked in a more concerted, integrated fashion to ensure food security. Table 1 highlights examples of progress to date and opportunities for improvements needed under changing climates. The table summarizes the relative complexity of selecting for, and maintaining, a new trait in a commercial breeding context, which informed application of new technologies may overcome. The key to this is envisaging how to create greater flexibility in the way crops respond to different environments and stacking traits additively, not antagonistically. Strategies also need to account for changes to seasons, planting times, the likelihood of specific climatic events, and which stresses are like to occur concurrently (Figure 2). Herein, we provide examples of new genetic technologies, traits, and stress-signaling components with an emphasis on the whole of the system. We believe the following are exemplar traits ideal for the purposes of pre-breeding and assessment for future incorporation into structured breeding programs.
Table 1.
Brief summary of proposed traits for target climate constraints and their value and utility in crop improvement and breeding.
| Climate constraint | Trait(s) | Value proposition? | Genetic control? | Genetic variability available? | Ease of selection | References |
|---|---|---|---|---|---|---|
| Frost/heat | Grain number (fertility), grain size | Unknown- High? | Complex | No | Difficult | Barrero et al. (2020) |
| Frost/heat | Rate of seed-fill | Unknown-High? | Complex | Yes | Difficult | Bruckner and Frohberg (1987) |
| Heat | Leaf architecture/orientation | Unknown-Small? | Largely simple | Yes | Largely simple | Hunt et al. (2018) |
| Heat | Canopy temperature | Unknown-low? | Complex | Yes | Simple | Amani et al. (1996) |
| Heat | Photosynthesis | Unknown-High? | Complex | Some | Difficult | Kromdijk et al. (2016) |
| Heat | Respiration | Unknown-Small? | Complex | No | Difficult | Posch et al. (2019) |
| Heat | Development | Unknown-High? | Simple | Yes | Simple | Rezaei et al. (2015) |
| Heat | Tillering/biomass | Unknown-High? | Complex | Some | Difficult | Houshmandfar et al. (2020) |
| Drought | Many (e.g. WUE, WSC, VPD-responsiveness) | Unknown-High? | Complex | Yes | Difficult | Lopes et al. (2015) |
| Drought | Root size/ architecture | Unknown-Small? | Complex | Yes | Difficult | Ober et al. (2021) |
| Drought | Coleoptile length | High | Simple | Yes | Simple | Zhao et al. (2022) |
| Drought | Early vigor | High | Complex | Yes | Simple | Rebetzke et al. (2004) |
| CO2 | Grain yield/protein | Unknown-High? | Complex | Some | Difficult | Bloom and Plant (2021) |
The value proposition reflects the cost–benefit of trait adoption to the breeding company; genetic complexity represents the number of genes, their genetic control, and reliable expression; genetic variability represents the availability and form of target genes, and access for breeding; and ease of selection represents confidence in the identification of target traits through marker-based selection or genomic prediction, and/or phenotyping for breeding.
Figure 2.
Overlap of extreme conditions and increased likelihood of contrasting abiotic stress events. A, As the likelihood of late cold events and early heat events begin to overlap, along with the increased risk of extreme rainfall or water deficits, so too does the likelihood of multiple, contrasting stress events occurring together or in quick succession. B, In order to mitigate the risk of these potential extreme events, efforts both to expand the range of conditions under which crops can yield effectively and provide resilience when those conditions are exceeded are now necessary. Created with BioRender.com.
Optimizing yield stability
An alternative to focusing on single abiotic traits is in the selection of crop traits that provide benefits to both current and future environments. Examples include altered crop phenology (Rezaei et al., 2015), greater coleoptile length (Zhao et al., 2022), higher rates of seed-filling (Bruckner and Frohberg, 1987), and greater early vigor (Rebetzke et al., 2004) all benefiting crop adaptation.
Crop establishment and early leaf area development
Optimal and timely plant establishment is critical in rain-fed cropping systems. In many parts of the world, crops are sown on the first rains, and water for growth is supplied as current rainfall. In Australia, variability in the timing, size, and frequency of rainfall events has increased over the past 30 years, and is contributing to uncertainty and even delays with the “sowing breaks,” autumn rains that initiate germination and seedling growth (Scanlon and Doncon, 2020; Flohr et al., 2021). In turn, predicted delays in seedling growth of up to 6 weeks have the potential to reduce wheat yields by up to 46% (Zhao et al., 2022).
Well-established crops provide ground cover to protect ameliorated soils, reduce water loss through soil evaporation, and increase crop competitiveness with weeds (Nelson et al., 2022). Breeding for faster leaf area development should reduce soil evaporation to increase crop WUE and yield (López-Castañeda and Richards, 1994), and competitiveness with weeds (Zerner et al., 2016; Hendriks et al., 2022a). Greater early vigor may also benefit crops through increased root growth early in the season (Palta et al., 2007; Hendriks et al., 2022a), while improved light interception should increase crop growth rate, biomass, and grain yield for late-sown wheat crops or in environments where crop duration is shorter (Regan et al., 1997). Interestingly, the rooting depth of wheat plants can be indirectly scored in trials using canopy temperature (also used as an indicator of WUE in leaves) as a proxy; low temperatures late in the day are well correlated with deeper roots (Li et al., 2019). Early emergence also increases the yield potential of crops through increased duration for root growth, tillering, and building of crop biomass while ensuring that crop development coincides with conditions optimal for growth and flowering and avoiding hot, dry conditions late into grain filling.
Genetic vigor is a target of many crop breeding programs (e.g. Richards et al., 2002), yet it relies on suitable genetic variation, repeatable target phenotypes, and confidence in selection, particularly in early segregating generations. In wheat, seed embryo size is strongly associated with increased seedling leaf width (López-Castañeda et al., 1996) and has been used as a target trait in S1:2 recurrent selection to increase early vigor (Zhang et al., 2015). Parental germplasm with upward increase of 30% in seedling leaf area has been used in the development of wheat breeding lines containing greater nutrient uptake (Ryan et al., 2015) and weed competitiveness (Hendriks et al., 2022b).
The shorter coleoptiles associated with the Green Revolution dwarfing genes, Rht-B1b and Rht-D1b, in modern wheat varieties limit sowing depths to <100 mm. The use of new dwarfing genes can increase coleoptile length to 150 mm, allowing for sowing into deep moisture following summer rain or long fallows. The greater coleoptile lengths also permit deeper sowing with increasingly warmer soils into future climates or where sowing early (Rebetzke et al., 2016). Yield benefits with deep sowing of long coleoptile wheat varieties have been predicted at 18%–21% and an estimated benefit of AUD$2.3 billion annually (Zhao et al., 2022).
Resource acquisition and transport
Engineering crops to maximize the exploitation of resources is a key directive for achieving progress in overcoming abiotic stress and increasing crop yields (Whitmore and Whalley, 2009). Abiotic stresses such as droughts, floods, and salinity make it challenging for crops to efficiently acquire resources. Ideal soil moisture levels serve dual critical roles by providing water as an essential resource and as a carrier of nutrients (White and Brown, 2010). This means that water limitation additionally restricts productivity by limiting nutrient acquisition. Specifically, drought and salinity each restrict nutrient uptake, whereas where there is an excess of water in flooded conditions the limitation in oxygen restricts root respiration, which is required to power root nutrient uptake mechanisms (Aguilar et al., 2003; Najeeb et al., 2015).
Examples of crop engineering targets relevant to improving resource acquisition and use include manipulating morphological targets such as optimizing root system architecture (Lynch, 2013), altering resource distribution between cells and tissues (Bush, 2020), manipulating root cell wall properties and membrane transport mechanisms and associated signaling pathways implicated in regulating nutrient acquisition and transport (Ogden et al., 2018), and optimizing beneficial root–microbe interactions (Farrar et al., 2014).
Photosynthesis and respiration in future climates
The potential for increasing yield by increasing photosynthesis has been heavily reviewed (Reynolds et al., 2012; Evans, 2013; Parry et al., 2013; Long et al., 2015; Simkin et al., 2019). Herein, our focus is on the interaction of photosynthesis and environmental perturbations. Fluctuations in light intensity are well established to impact photosynthesis and protective mechanisms, such as nonphotochemical quenching, with recent efforts to optimize energy dissipation demonstrating the potential for yield improvements (Leonelli et al., 2017; Gabilly et al., 2019; Steen et al., 2020). However, as noted above, earlier planting can lead to an increased risk of frosts, which in conjunction with sunlight is a severe oxidative stress, and later in the season, drought, heat, and light all impair the photosynthetic performance, with combinatorial impacts being more than additive (Mittler, 2006).
In this respect, it is unlikely that targeting individual components of the photosynthetic apparatus will be of substantive benefit under stressed, resource-limiting growth conditions. In chamber-grown rice, modest growth and yield benefits under sustained elevated temperatures have been achieved by increasing Rubisco activase thermostability (Scafaro et al., 2018), though untested in the field. Rather than a single target approach, an integrative methodology to model the various interactions in response to photosynthesis, such as Wu et al. (2019), is appropriate. Wu et al. (2019) identified several targets in C3 and C4 species by incorporating biochemical models of photosynthesis into the crop development model system agricultural production systems simulator (APSIM) (Holzworth et al., 2014). Such a strategy would be invaluable in dealing with many of the complex abiotic response traits dealt with in this document (Hammer et al., 2010).
Other integrative targets for improvement are the retrograde signaling pathways for which the chloroplast acts as an environmental sensor (Chan et al., 2016a). Tuning such retrograde signaling pathways (with input from integrative modeling methods as above) may provide a feasible mechanism whereby manipulation of a single (or few) gene(s) optimizes entire gene networks capable of stimulating integrated cellular and whole plant response to oxidative stresses, including drought, temperature, and light. Such a discovery would be of incredible scientific and agronomic value as it would give insights into how to better tune resilience–yield tradeoffs (see below).
The contribution of optimizing respiration to improve crop productivity has been overshadowed compared to the global efforts on photosynthesis (Reynolds et al., 2021). The impact of genetic and functional variation in respiration on growth has been reported for a few crops, including wheat (Winzeler et al., 1988; Pinto et al., 2017). This area is an example of “low-hanging fruit” for yield research, as inefficient respiration can “burn” energy captured by photosynthesis (Bouma et al., 1994), and in general respiration rates are negatively associated with growth and yield (Heichel, 1971; Wilson and Jones, 1982; Winzeler et al., 1988; Hauben et al., 2009).
What is of particular interest from a future climate perspective is that cereal yields are more strongly correlated with night temperature than day temperature (Ziska and Manalo, 1996; Cheng et al., 2009; Gimenez et al., 2021). Night temperature effects on rice yield have been well investigated along with overall temperature changes (for a summary of evidence, see Xiong et al., 2017). Dark respiration rates can increase with short-term increases in night temperature (high-temperature events), but acclimation to more constant increases also occurs (Atkin and Tjoelker, 2003; Posch et al., 2019). Under acclimation, although overall O2 consumption declined with consistent night temperatures, alternative oxidase activity/capacity (a secondary, low-efficiency form of respiration) increased, which may reflect the reduced efficiency of adenosine triphosphate (ATP) production at night (Posch et al., 2022). Together this indicates that genetic variation in night-time respiration may be a significant determinant of productivity and requires further research as to the impacts of climate change.
Optimizing climate resilience
Drought
As a consequence of the yield–resilience tradeoff, the development of drought-tolerant crops has long been a “holy grail” for breeders and plant scientists over the past 50 years. Indeed, well before concerns with climate change were first aired, significant efforts had been placed into understanding the morpho-physiological basis of adaptation to drought in rain-fed and water-limited environments. This is because plant responses to water deficit occur on multiple, coordinated levels, combining phytohormones, reactive oxygen species, hydraulics, calcium waves, and hormone-like peptides for both short- and long-distance signal propagation (for review, see Takahashi et al., 2020). Initial signals for drought stresses in plants are derived from the roots, which react to prolonged water deficit by often increasing growth (Sharp, 2004; Xiong et al., 2006). Root hydraulic changes in response to soil water deficit are transmitted to the shoots and leaves, causing reduced leaf water potential (Christmann et al., 2013). This leaf water potential in turn causes a reduction in turgor, a mild increase in solute concentrations, and triggers the biosynthesis of the phytohormone abscisic acid (ABA) (Pierce and Raschke, 1980).
The accumulation of ABA during drought (Trivedi et al., 2016) results in both short-term drought responses, such as stomatal closure to reduce water loss via transpiration, and long-term changes to growth and development, such as root cell elongation to enable plants to recover more efficiently from water deficit. The central role of ABA in drought responses and plant development can be illustrated best by the number of signaling pathways ABA functions within, such as stomatal aperture response (Daszkowska-Golec, 2016), modifying root architecture (Harris, 2015), and interacting with multiple other plant hormones (Hussain et al., 2019) (for a comprehensive review of ABA-mediated drought stress response, see Muhammad Aslam et al., 2022). Given such wide-ranging responses to ABA, targeting this system for improved drought tolerance will require a systematic approach utilizing synthetic biology approaches (Park et al., 2015; Vaidya et al., 2017).
Past efforts at improving ABA-based drought tolerance have been included focusing on areas such as ABA sensitivity by overexpressing ABA receptors (Yang et al., 2016; Nuccio et al., 2018); this increased WUE of Arabidopsis under water-deficit conditions and reduced transpiration rates, but such over-sensitivity to ABA also lead to reduced plant stature. The use of a chemical ABA agonist which selectively targets ABA receptors, blocking PP2C inhibition of SnRK2 activity, was able to reduce transpiration rates and improve drought tolerance in Arabidopsis, and prevented wilting in detached leaves of barley, soybean, and maize (Okamoto et al., 2013); these results indicate the need to target individual receptors in a nuanced, measured way. It also demonstrates nongenetic measures that may be taken within a field season to prepare crops for predicted, oncoming drought conditions via chemical analogs. However, a consistent finding is that reduced transpiration rates produced by targeting ABA action naturally lead to lower yield, especially given that high transpiration is often used as a marker of superior yield in field experiments, even under drought. There cannot be a tradeoff of resilience in drought for a reduced yield in good years (Tuberosa, 2012; Nuccio et al., 2018).
Heat
For many crop species, temperature stress has a greater influence on reproductive than on vegetative development, with yield loss due to temperature largely associated with abnormal inflorescence development and reduced fertility (Zinn et al., 2010). However, plants can adapt to gradual increases in temperature by modifying a suite of developmental traits, such as flowering time, inflorescence shaping, and male reproduction (Prusinkiewicz et al., 2007; Yu et al., 2017; Li et al., 2021).
Although several key sensors and regulators in the perception of temperature and thermo-morphogenesis have been identified (Jung et al., 2016; Sureshkumar et al., 2016; Casal and Balasubramanian, 2019), the factors that influence organ number, identity, and function in response to temperature remain largely unknown. Recent advances in identifying factors involved in the maintenance of cereal fertility with temperature increases include the identification of a MADS-box protein integral to normal cereal inflorescence shaping (Li et al., 2021) and a receptor-like kinase that maintains male fertility (Yu et al., 2017). The adaptive expression of the genes necessary for these features in response to temperature is a developing area of research aiming to improve yield stability with temperature change.
Severe heat stress often causes transient unfolding of heat-labile proteins, damaging protein quality control machineries, such as molecular chaperones and proteasomes. Manipulation of molecular chaperones, such as heat shock proteins (HSPs), may facilitate the refolding of such heat-misfolded proteins, reverting them to functional native proteins. Loss-of-function mutants of molecular chaperones in model plants and crops have been found to have increased sensitivity to heat stress, while overexpression of metabolic enzymes and HSPs slightly reduces this sensitivity (Lee et al., 2007; Guan et al., 2013; Lin et al., 2014; Chen et al., 2019b). Such findings confirm the crucial roles of molecular chaperones in protecting and repairing heat-induced thermolabile proteins (Cabrera et al., 2020), and also indicate that heat tolerance in plants is a polygenic trait, combining chaperones and metabolic enzymes. These enzymes produce reactive oxygen species (ROS)-scavenging and thermo-protective metabolites, heat-induced signal proteins, and transcription factors (Ding et al., 2020), as evidenced by recent transcriptomic, proteomic, and metabolomic data (Chen et al., 2019b; Ran et al., 2020; Zheng et al., 2022). Chaperones and their metabolic enzyme allies provide an important and obvious target for future molecular engineering of heat stress tolerance for yield stability.
Cellular membranes are also vital in defense from heat stress. The plant cell membrane represents the first line of heat stress defense, and such stress can directly modify the integrity and plasticity of cellular phospholipid membranes. Changes in lipid composition and membrane fluidity are important for plant heat tolerance; such changes alter the structure and activity of membrane-localized proteins, as well as other ion or anion channel proteins (Ding and Yang, 2022). Elucidating heat signaling components, including important sensors, effectors, and transcription factors, will provide not only a comprehensive understanding of thermo-sensing, transduction, and regulation but also useful genetic targets for redesigning crop heat stress signaling pathways to obtain heat resilience crops to secure food security under changing climates. Given the range of components utilized by plants to sense and react to heat stress events, manipulating single genes or proteins is unlikely to produce heat-resilient crops for all conditions; rather, incorporation of these data into whole-plant models such as that of Wu et al. (2019) would be of value to identify the combination of traits needed to be modified to this end (Hammer et al., 2010). In their excellent review of breeding needs and opportunities for climate-related plant adaptation, Chapman et al. (2012) conclude that adaption to high temperature is the greatest priority for crop breeding in the Australian context, given the greater reliability that increased temperatures will predominate, as opposed to highly fluctuating and extremes of rainfall, frost, or other climatic stresses.
Crop hydraulic conductance and salinity tolerance
Engineering crops to secure productivity under future projected climatic conditions will involve optimizing their hydraulic properties. High root hydraulic conductivity can enable crops to quickly take advantage of rainfall events but can be problematic in dry conditions when water needs to be retained for use during reproductive growth stages. Adjustments to root hydraulic conductivity have been made by selecting smaller xylem vessel diameters in seminar roots to decrease axial conductance and reduce the risk of air embolism (Richards and Passioura, 1989; Richards et al., 2002; Hendel et al., 2021). Under terminal drought conditions, wheat with lower root axial conductance delivered greater grain yields when compared to isogenic lines with similar biomass and architecture but higher axial conductance (Hendel et al., 2021). Hydraulic conductivity is also controlled by differential regulation of the subsets of aquaporins that influence cell membrane permeability to water and solutes (Tyerman et al., 2021). Aquaporin function influences root water uptake and impacts plant transpiration rate by influencing phloem loading, xylem water exit, stomatal aperture, and gas exchange (Ermakova et al., 2021; Shivaraj et al., 2021). Manipulation of the regulation and abundance of aquaporins is regarded as a promising strategy for engineering crops with greater tolerance to water-limited conditions (Sinclair et al., 2017; Chen et al., 2021; Patel and Mishra, 2021).
Plant choices for salt exclusion
All plants exclude >95% of soil sodium (Na+) from their tissue. A significant focus has been to investigate enhancing Na+ exclusion from the leaf blade, the site of photosynthesis, to enhance yield. This is in part because of the ease of measuring leaf Na+ and the early observations of yield negatively correlated with leaf Na+ concentration in certain plants and crops (soybean, grapevine, durum wheat) (Munns and James, 2003; Byrt et al., 2007; Munns et al., 2012; Guan et al., 2014; Henderson et al., 2018). However, the influence of Na+ exclusion on improving yield in other crops/plants has been inconsistent and has led to the successful release of only a few commercial cultivars. Accessions of bread wheat, barley, and Arabidopsis have recently been found to accumulate high concentrations of leaf Na+ without a penalty in shoot biomass or yield (An et al., 2017; Busoms et al., 2018; Borjigin et al., 2020; Houston et al., 2020)—in some cases, there are reports that a moderate accumulation of Na+ in leaf tissue can improve biomass production (Genc et al., 2019).
A member or members of the HKT gene family in many species are frequently identified as key for controlling leaf Na+ accumulation, often accounting for >50% of the source of variation (Byrt et al., 2007; Møller et al., 2009; Mian et al., 2011; Babgohari et al., 2013; Byrt et al., 2014; Garcia-Abellan et al., 2014; KobayaShi et al., 2017). The incorporation of the Nax1 and Nax2 loci in durum wheat (Triticum turgidum subsp. durum), which are linked to TmHKT1;4 and TmHKT1;5, respectively, from an introgression (Triticum monococcum; James et al., 2006) improves Na+ exclusion and yield in the field (James et al., 2012; Munns et al., 2012). However, disruption of the bread wheat (TaHKT1;5, Triticum aestivum) and barley (HvHKT1;5, Hordeum vulgare) gene, while leading to increased leaf Na+, is not linked to a biomass/yield penalty (Borjigin et al., 2020; Houston et al., 2020; Huang et al., 2020; Borjigin et al., 2021). Some ecotypes of Arabidopsis, which is typically classified as salt sensitive, can possess a high shoot Na+)—accumulating allele of AtHKT1;1HLS, and be better adapted to intermediate–high soil salinity levels (Baxter et al., 2010; Busoms et al., 2018). There are Arabidopsis ecotypes that lack a functional HKT in the roots and are susceptible to salt, which can be restored through complementation (Møller et al., 2009) which, as widely found for multiple plant species, demonstrates that response to salinity is a complex, multi-component trait involving multiple genes (Asif et al., 2018; Luo et al., 2019; Abdelraheem et al., 2021; Asif et al., 2021; Fan et al., 2021; Venkataraman et al., 2021). The relative salinity tolerance of ecotypes expressing HKT in the roots or shoots hints that developmental stage and tissue might be important. With the reproductive stages being very sensitive to salinity, the transfer of salt to the inflorescence may well be an important component of salinity tolerance regardless of where the point at which salt exclusion occurs, the leaves or the roots (An et al., 2017; Busoms et al., 2018). Ecotypes of Arabidopsis with poor shoot exclusion of Na+, such as Tsu-1, were found to have high expression of AtHKT1;1 in stems, reducing the accumulation of Na+ in floral tissues, and had greater fertility than those ecotypes which had low stem AtHKT1;1 expression but good leaf exclusion (An et al., 2017).
Rather than a “one shoe fits all” solution, Na+ exclusion from the leaf may be a strategy that works well in high rainfall locations, where there is little water limitation and Na+ is excluded to avoid any damage to the plant. However, in low rainfall areas, where water is limited, a greater concentration of Na+ in leaf tissue will help lower leaf water potential and assist with the uptake of water from the low rainfall environment, promoting growth. In this scenario, the plant walks a tightrope of allowing enough Na+ to accumulate to bring in more water for growth, and too much Na+, which leads to disruption of metabolism and premature senescence. In barley, the high Na+ accumulating HvHKT1;5 allele appears to have been selected for in breeding programs, increasing in frequency from 4% in landraces to 35% in elite cultivars (Houston et al., 2020). This is hypothesized to be due to Na+ being used as a substitute for K+ as an abundant osmolyte to reduce leaf water potential and improve water uptake in North-West Europe’s low K+-containing soils (Houston et al., 2020). Similarly, Arabidopsis ecotypes possessing a high shoot Na+ accumulating allele of AtHKT1;1HLS may be better adapted to intermediate–high soil salinity levels due to their ability to substitute Na+ as an osmolyte to replace K+, which can be difficult to uptake when Na+ is abundant (Baxter et al., 2010; Busoms et al., 2018; Venkataraman et al., 2021).
The range of salt exclusion responses possible from the different HKT alleles provides considerable power for future salinity amelioration methods. Using stress response elements, it will be possible to engineer a switch that turns on or off the HKT gene in crop plants when water is limited or at certain times of development. Evolutionary or mixture populations could be used in place of single homogeneous cultivars (Ceccarelli and Grando, 2020); several iterations of a background cultivar could be produced with differing HKT1 alleles, ranging from low shoot salt accumulating through high accumulating. Depending on the year and environmental conditions, selection pressures will favor the most appropriate salt exclusion method, preserving yield. These selection pressures will vary across fields with salt and water levels, not just by field season. Alternatively, targeting the tissue specificity of HKT expression, similar to what had been done in roots (Møller et al., 2009; Plett et al., 2010), should also be investigated as a mechanism to protect floral tissues.
Technology toolkits needed for future climates
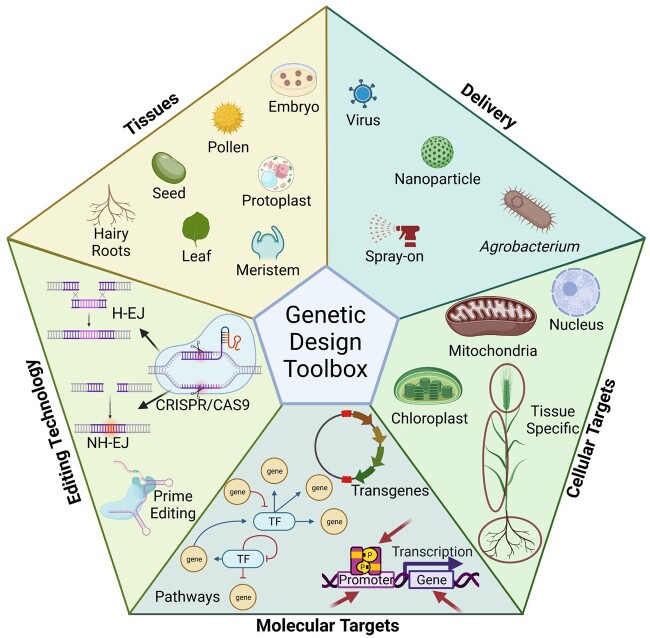
Given the need for rapid crop improvement to meet climate challenges, it is appropriate to cast the net wider and take advantage of new technologies and approaches in addition to current breeding efforts. Targeted approaches to tailoring abiotic stress responses, introducing genetic diversity in the form of alleles from other plant species, tissue-specific editing of genes, alteration of expression, and translation are just some of the methods that will be utilized to meet these challenges (Figure 3). Below we describe and assess the utility of the new technologies and provide examples of how they could revolutionize our responses to our changing world.
Figure 3.
Genetic Design Toolbox. The modern range of genetic tools available to combat extreme abiotic stress events, including a variety of tissues to produce new, modified materials utilizing diverse methods of delivery, targeting tissues and subcellular compartments by modifying individual transcription factors and genes, introducing multiple novel transgenes, through entire pathways, with the latest in gene-editing systems. Created with BioRender.com.
The CRISPR–Cas9 led gene-editing revolution in crop plants
Gene editing in plants has now been widely applied using the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) system (Zhang et al., 2019). The appeal here is editing the plant’s own genes rather than introducing foreign genes. The simplest such approach is the use of guide RNA (gRNA) to introduce a double-strand break (DSB) in a precise, targeted manner. This DSB is then repaired by an efficient, but imprecise, nonhomologous end joining (NHEJ) mechanism resulting in random nucleotide insertions/deletions, representing a tool that mainly generates loss-of-function mutations (Chen et al., 2019a). As such, NHEJ approaches have been used to eliminate undesirable traits, and no doubt there will be many opportunities for its use in increasing abiotic stress resistance in crops.
Precise genome editing is achieved via homology-directed repair (HDR). Here, the CRISPR/Cas9 reagents and a donor DNA template with the desired gene edit are simultaneously delivered into the cell. Upon the creation of the DSB, the template is used to repair it via HDR (Chen et al., 2019a). This can result in sequence replacement or insertion to create knockin mutations that are able to introduce new genes and/or gain-of-function traits. However, the efficiency of the HDR process is poor, and methods of delivering the DNA template into the plant cell at the required concentration are limited (Chen et al., 2019a, 2022). Nevertheless, there have been examples such as the delivery of template DNA and reagents via particle bombardment into immature maize embryos. One example is the generation of novel variants of a negative regulator of ethylene response, the ARGOS8 gene, in which its level of expression was increased, resulting in the improved tolerance of maize to drought (Shi et al., 2017); ethylene response genes formed a large part of the field-validated beneficial genes found in Simmons et al. (2021), including ARGOS1 and other ARGOS genes (Guo et al., 2014; Shi et al., 2015). Use of gene editing to interrupt the expression of the rice ENHANCED RESPONSE TO ABA1 (OsERA1) gene produced lines with increased root growth, enhanced ABA sensitivity, and some improved drought resistance (Ogata et al., 2020). Precise editing of a tomato (Solanum lycopersicum) multi-stress responsive gene, SlHyPRP1 (a member of the hybrid proline-rich protein family) was attempted using multiple gRNAs to target different functional elements of the gene in question, demonstrating a mild salt-tolerance phenotype in seedlings (Tran et al., 2021).
Base-editing approaches for precision genome editing
Given the difficulties of introducing donor DNA into cells, approaches have been devised to circumvent this need. First, is the development of base editors (BEs), an adaptation of the CRISPR–Cas9 approach (Molla et al., 2021). Here, an inactive Cas9 protein is fused to a deaminase nucleotide-modifying enzyme leading to the generation of cytosine BEs (CBEs), that can perform C to T transitions (Komor et al., 2016), or Adenine BEs (ABEs) that can perform A to G base transitions, and both are able to efficiently generate nucleotide variants at targeted loci (Kang et al., 2018). Building on this was the development of dual BEs, by creating a Cas9 fusion protein encompassing both CBE and ABE activities, referred to as saturated targeted endogenous mutagenesis editors (STEMEs); however, this did not result in transversions, insertions, or deletions (Li et al., 2020). This issue was circumvented with the innovation of prime editors (PEs), where a modified Cas9 is fused to reverse transcriptase and a prime-editing gRNA, which not only targets the Cas9 fusion to the desired locus but also acts as a template from which precise edits are incorporated into the DNA, and therefore unlike BEs are not restricted to what base changes can be made (Lin et al., 2020).
To date, for many of these approaches there have only been limited reports on their applications; no doubt there will be further rapid innovation to remove technical limitations and low efficiencies, such as the use of alternative natural or modified Cas nucleases, for which there is an abundant choice, making these approaches standard in the plant breeders toolkit. Such emerging approaches include CRISPR-inducible gene editing, tissue-specific gene editing (CRISPR-TSKO), and epigenome editing, all of which have the potential to contribute to increasing the tolerance of crops to abiotic stress (for review, see Chennakesavulu et al., 2022).
Advanced gene editing for agronomic trait development
CBEs, ABEs, STEMEs, and PEs can all be used for generating a diversity of target loci in situ, resulting in the capacity of directed evolution in planta. For example, a region of the acetyl coenzyme A carboxylase (ACC) gene was targeted for near-saturated mutagenesis via STEME in rice, and gene-edited variants in regenerated plants could confer herbicide tolerance (Li et al., 2020). A subtler approach is the modification of regulatory elements. One promising avenue is the modification of upstream open reading frames (uORFs), enabling modification of the translational output of a gene via uORF editing (Zhang et al., 2018). Furthermore, could be “promoter editing,” an approach that could result in the generation of diverse cis-regulatory alleles. For instance, targeting the cis-motifs in promoter regions of genes control major traits generated a variety of alleles with different expression levels resulting in a continuum of trait variation that could be utilized for quantitative breeding (Rodriguez-Leal et al., 2017; Liu et al., 2021). As both abiotic cis-regulatory elements and uORFs play roles in abiotic stress (Causier et al., 2022), the gene editing of these elements would likely be able to modify how these abiotic stress response genes are regulated at the transcriptional and translational levels. This could be applied to key abiotic stress loci, to fast-track the generation of variants at these loci, and then be tested to assess their tolerance to stress.
Technologies to remove crop transgenesis as a research bottleneck
Most crop transformation protocols are far from routine and remain the limiting factor regarding crop gene technology research and development. For most species, the approaches are dependent on decades-old tissue culture methods that are complex, time-consuming, and labor intensive. Furthermore, efficient transformation protocols are hard to reproduce within and between species. Moreover, the protocols create unintended mutations and are varietal dependent, where many are not easily adapted to elite germplasm. This has rendered the technology more of a pre-breeding “proof-of-concept” exercise, rather than being utilized as a direct breeding tool. All these factors have limited gene-technology research in crops, both at the university and industry levels.
Optimizing a transformation protocol requires the assessment of multiple factors, including target plant tissue, the Agrobacterium strain, co-cultivation conditions, tissue-culture gelling agents, hormones, nutrients and other chemicals, the binary vector system, and selection gene among others. All these factors need to be optimized to be able to result in a high plant transformation efficiency (Hayta et al., 2019). Boosting transformation frequencies has been achieved by the expression of master developmental regulators (DRs), such as Baby boom (Bbm) and Wuschel2 (Wus2), whose expression can produce high transformation frequencies in maize (Lowe et al., 2016), or GROWTH-REGULATING FACTOR genes that could boost efficiencies in a variety of monocot and dicot species (Kong et al., 2020). These DRs have been subsequently used for the development of a tissue culture-free transgenesis protocol, a potentially revolutionary step for crop transformation. Here, infiltration of DRs directly into Nicotiana benthamiana plants could trigger the de novo induction of meristems from which regenerated transgenic plants could be obtained (Maher et al., 2020). Additionally, co-infiltration of gene-editing reagents could result in gene-edited plants, and in some instances these gene-edited plants did not contain a transgene, implying that transient expression was sufficient for gene editing (Maher et al., 2020). Given the conserved fundamental nature of DRs, such protocols potentially could be adapted to other species, and so far, this has been shown in tomato, potato, and grape (Maher et al., 2020). Further development and routine adoption of this tissue culture-free transgenesis and gene-editing approach promise to remove current crop transformation bottlenecks.
An alternative method to gene editing without tissue culture is the use of RNA viruses. Although they generally have a limited cargo capacity, the approach has been to incorporate gRNAs into viral genomes, which are then infected onto a pre-existing Cas9-expressing plant. However, very few heritable events were achieved due to a lack of germline infection (Ali et al., 2015). Solving this was to fuse the gRNA to a mobile RNA such as from Flowering Locus T (FT) which transports the gRNA to the meristem, resulting in a high frequency of heritable gene-editing events (Ellison et al., 2020). This proof-of-concept has been achieved in N. benthamiana but could be easily applied to other species where only a single Cas9 transgenic plant is needed for the implementation of the strategy.
Most of the above examples require stable integration of the transgenes encoding the gene-editing reagents. To avoid this, non-DNA approaches have been developed, such as preassembling Cas9–gRNA ribonucleoproteins (RNPs) that can be delivered into plant cells. This has been achieved in maize and wheat via gold-particle bombardment of immature embryo cells (Svitashev et al., 2016; Liang et al., 2017), or in Arabidopsis, tobacco, lettuce, and rice via transfection of protoplasts (Woo et al., 2015). In all these cases, mature gene edit plants were regenerated at efficient frequencies. One advantage of transiently expressing the gene-editing reagents is that less off-target effects occur in comparison to the long-term expression of reagents via an integrated transgene (Liang et al., 2017). This was also found to be the case for BEs, where a CBE induced many gRNA-independent nucleotide alterations throughout the genome of rice (Jin et al., 2019), but less so when transiently expressed. Additionally, a transient expression or delivery would be preferable in a regulatory sense, as no transgene integration into the genome avoids the associated regulatory issues. However, these approaches are likely limited to only a few species, as the regeneration of plants from embryo cells and protoplasts remains challenging.
An alternative strategy is the use of nanoparticles to deliver CRISPR–Cas cargo into plant cells (Demirer et al., 2021). Here, nanoparticles can protect cargo from degradation, and potentially deliver the cargo in a species/varietal-independent manner, potentially making this method species agnostic. Other issues that nanoparticles could solve include enhancing the delivery of donor DNA into recipient cells for increasing the efficiency of HDR editing, or specifically targeting germline cells, to ensure the gene editing is heritable (Demirer et al., 2021).
Spray-on RNA as a dynamic approach for managing field-grown crops
An issue of breeding for crop abiotic stress tolerance is that there is no guarantee as to which abiotic stresses may be encountered during any given season. Sowing a drought-tolerant crop in a wet season may be counterproductive, especially if there is a yield penalty associated with the abiotic-tolerance trait. An alternative approach would be to modify gene expression in field-sown crops to respond to the stress that they are encountering. Topical RNA application to transiently alter gene expression of a sown crop offers this potential and is currently an area of great interest for its translational potential in the field (Rank and Koch, 2021; Torti et al., 2021). Of further benefit, RNA applications circumnavigate the need for generating and commercializing a transgenic crop, and so avoid these regulatory issues.
It has now been known for some time that double-stranded RNA (dsRNA), short-interfering RNA (siRNA), and single-stranded RNA (ssRNA) could all trigger efficient silencing in plants via exogenous application, which is then absorbed and able to move systemically throughout the plant (Sammons et al., 2011; Koch et al., 2016). Methods to deliver the in vitro synthesized RNAs into plant cells have improved via modifications such as using surfactants (Silwett-77) or high-pressure spraying (Dalakouras et al., 2016). Moreover, nanoparticles have been investigated for their ability to deliver cargo into plant cells, overcoming the cell wall barrier. There is a wide range of options with different characteristics based on the need in the application; silica nanoparticles, carbon nanotubes, and gold nanoparticles, all of which are in their early days of development with much to learn about their optimization to deliver RNA cargos (Zhang et al., 2022). The use of layered double hydroxide (LDH) nanosheets that bind dsRNA (LDH–dsRNA complexes are termed BioClay), which enables a slow, sustained release of the dsRNA into the plant cell resulting in a prolonged transient knockdown of a target gene, shows great promise (Mitter et al., 2017). Moreover, being nontoxic, water-proof, and degradable appear to have clear practical applications in terms of crop management.
Technology to assist when amelioration fails
Additional management changes to deal with abiotic stresses will be needed as the likelihood of extreme stress events damaging primary crop yield increases. It is likely that, even with the amelioration of stress or improved use of stress responses to counter environmental fluctuations, crop losses will continue or increase. In such cases, the secondary use of crops to ensure value to farmers and to reinforce food security will become more prevalent. A common farm management technique for failing winter crops (due to drought, frost, etc.) is to allow for grazing or the harvest of biomass for hay, fodder, or silage production (GRDC, 2018; Piltz et al., 2021). Maximizing the value of these salvage crops requires harvesting at defined times to maximize digestibility and metabolizable energy content, along with protein levels. Typically, carbohydrates and nitrogen are remobilized to grain or seed post-anthesis, and ideal levels of all components vary with endpoint and species (Khorasani et al., 1997; Nadeau, 2007).
Engineering “smart plant” varieties with the ability to use accumulated abiotic stress signals to determine in planta the likelihood of primary productive yield may improve secondary harvest capabilities. When conditions do not support conventional harvesting, such plants could inhibit the normal remobilization of leaf and stem proteins and carbohydrates to seed, and maintain optimal nutrient content for such fodder production. Drought signaling mechanisms such as the chloroplast retrograde signaling SAL1–PAP pathway (Estavillo et al., 2011; Chan et al., 2016b; Phua et al., 2018) could be used as a means of identifying drought stress in plants by creating PAP concentration-dependent signals in leaves and signaling their presence using leaf color changes. Using Ca2+ or ROS sensors specifically expressed close to plant anthesis could provide a similar signaling mechanism to indicate the likelihood of cold/frost stress that will impact yield potential (Pareek et al., 2017). Such mechanisms could provide farmers with improved, technology-free means of rapidly assessing crop health and the likelihood of value in the primary harvest.
Public engagement and regulation
As argued above, new approaches are required to meet the challenges presented for crop resilience, especially in the face of dramatic climate change and its flow-on effects. However, technologies developed in response to this crisis must be considered in the context of their likely social reception and uptake. If farmers are not willing to grow these crops, or consumers are not willing to buy and eat products made from them, even the most scientifically compelling technologies will be unlikely to proceed to market.
Limited research has been undertaken about public participation and engagement in the newest genetic technologies, particularly gene editing, and considerably more empirical research is needed. We reflect on lessons to date based on this limited evidence basis in relation to public engagement in and understanding of gene-edited crops, particularly in species intended for the food supply, and propose participatory approaches as central to fostering public engagement in this domain.
What do people think about genetically modified and gene-edited crops?
We have considerable empirical evidence on genetically modified (GM) crops, where there is a breadth of views reflected at the national and other levels. Research has shown that views on GM differ across various types of public settings, depending on perceptions of what the frame should be for considering GM (e.g. whether the precautionary principle is invoked), potential benefits in relation to risks, who stands to benefit, and the purposes for which GM is being used (Ankeny and Bray, 2016). Attitudes toward the use of biotechnologies in food production are more cautious and tend to be negative (Bray and Ankeny, 2017), for instance, in comparison to the use of biotechnologies in medical applications, in part because many consumers do not see benefits for themselves and their families from these types of applications. Systems for food labeling have been hotly contested due to what is seen by some as loopholes or gaps in the system particularly if consumers wish to know about GM processes used to make the product and not simply whether the final product contains GM (Bray and Ankeny, 2015). Critics contend that current labeling requirements fail to allow consumers to avoid the purchase and consumption of food made with GM components.
There is limited empirical information available specifically on public views on the uses of GM or gene editing to produce traits that result in more resilient crops. We do know that consumers and community members often have preferences for food that do not map directly onto the GM/non-GM distinction. Many prefer food that is “natural” (as defined as minimally processed, see Lockie et al., 2005), locally produced, healthy and nutritious, and additive-free, and the presence or absence of GM ingredients is not necessarily the main factor in food choice for many, though women with children tend to express more concerns about GM (Bray and Ankeny, 2017). In addition, many have concerns about GM and other biotechnologies that go well beyond the effects on the food supply, noting broader issues such as the effects on farmers of technologies that prevent seed saving, and the conflation of the technology with the consolidation of power and intellectual property by multinationals, and environmental impacts from the use of agricultural chemicals in farming systems in conjunction with GM (see also Deckers, 2005). Another study found that although benefits to farmers and consumers were not compelling, food security was thought to be a significant issue in developed countries (McFadden and Smyth, 2019).
The limited available evidence about attitudes to gene editing seems to indicate that the public attitude is slightly more positive than older forms of GM, and slightly more negative than food produced using traditional breeding techniques, with the “more targeted” and “less distant” nature of gene editing compared with more “random” and “distant” GM technique often considered more positively (Cormick and Mercer, 2017; Gatica-Arias et al., 2019; Kato-Nitta et al., 2019; Ferrari, 2022). However, it must be stressed that more evidence is required in this domain: it could be the case that this relative positivity toward gene editing appears as a result of the framing biases of the research thus far conducted (for a recent review of the literature on public understandings of new breeding techniques including gene editing, see Grant et al., 2021). In one of the few studies that did not define gene editing and other newer technologies as distinct from GM, participants were equally split on whether these techniques were similar or different in important ways (Debucquet et al., 2020). The limited data available do suggest that there may be some willingness to consider novel crops modified to be more resilient if they indeed benefit farmers (particularly those seen as family farmers as opposed to multinationals or corporates), and if the crop fulfills other beneficial desiderata such as reduction of agrochemical use or documentable environmental benefits, and if it is produced using gene editing rather than conventional GM. However, specific traits or products may be received in different ways by publics and communities, depending on their individual characteristics.
Is more education the answer?
Although it might seem initially attractive to rely on information provision of the best scientific evidence available to convince publics to be more accepting of crops engineered to be more resilient, empirical data suggest that more knowledge about science or agriculture does not necessarily generate more support for biotechnologies. For instance, Lea (2005) detected little difference in GM food beliefs between those with and without post-secondary school qualifications, and Wheeler (2009) documented different levels of support for GM crops among agricultural professionals in Australia. Bray and Ankeny (2017) found that women involved in GM crop development and those with health science training differed not only in their views on GM but also more importantly in how they considered and used evidence. Those in plant science thought that no evidence of harm from GM to date (in the scientific literature and in their own consumption experiences) served as a sufficient basis for them to be unconcerned, whereas women with health science training thought that a perceived lack of evidence of safety from testing of GM foods created unknowns that made them reject purchasing certain foods. What is clear from this data is that understanding of what counts as evidence, and when groups think that there is enough evidence to proceed to do research or commercialize GM products, does not always align with the standards associated with the science or regulation alone.
These findings are not surprising as it reinforces that what is known as the “deficit model” of science communication is highly problematic. Such a model relies on the idea that rejection of technology is due to an information deficit within the intended users/consumers of that technology (Gregory and Miller, 1998; Sturgis and Allum, 2004). Empirical work has shown that the underlying assumption, namely that providing more information will change people’s views on science and make them more positively inclined toward it or more trusting of it, is unsupported. Many studies show that polarization occurs among the publics following the provision of information toward both extremes of support or rejection (see Brossard and Nisbet, 2007; Hart and Nisbet, 2012). Information provision or communication alone also fails to promote important democratic values such as inclusion and engagement.
Thus although many tend to implicitly fall back on the flawed deficit model both in science and in science communication itself, where quantitative studies tend to reduce understanding or engagement to “knowledge” about the technology itself, best practices in science communication and public engagement have shifted away from this model. Attitudes toward novel biotechnologies including GM and gene editing should never be understood in isolation from broader issues, such as what makes good food and what our agricultural system should look like, and should not be assumed to be negative (on this last point, see Priest and Greenhalgh, 2011 on nanotechnology) when in fact they are complex and grounded in people’s understandings and experiences. Gene editing may present particular challenges, for instance regarding its lack of traceability, with some scholars arguing that this “advantage” could be considered the same as being unmonitorable, stealth, or potentially even insidious (see Pirscher and Theesfeld, 2018), and better practices for engagement and communication on this issue will require more rigorous research investigating the intersection of values and responses to gene editing, and fostering more deliberation about food production processes.
How can “social license to operate” be ensured in this space?
The concept of “social license to operate” (or SLO) is frequently utilized in the context of public engagement with agriculture and associated technologies. SLO is not a license in any formal sense: instead, it is an informal agreement granted by communities and relevant stakeholders in the case of research to scientists, a field, or an industry to be permitted to do research. Typically, SLO is discussed primarily when it is lost or comes under scrutiny and often is not actively cultivated until problems or concerns are noted.
Although social license in part can relate to making available technical and scientific knowledge about the potential for benefits or harms, including environmental, social, and economic factors, knowledge is only a small part of the overall picture, in part because the publics tend to view these issues as deeply interconnected. Other components which are important to fostering SLO include the reputation of the organization doing the research or promoting the product, the level of transparency with which they operate, and whether the organization is trusted (Rooney et al., 2014). In the domain of genetic technologies, there are diverse regulatory frameworks across the globe that rely on different definitions for when a crop is classified as GM, gene edited, or traditionally bred, despite calls for more clarity and consistency about definitions of gene editing in association with regulation (e.g. Bartkowski et al., 2018; Bartkowski and Baum, 2019; and Nature, 2021). Various regulatory systems are also viewed as having differing levels of efficiency; for instance, the European Union’s (EU) gene technology regulatory framework was recently reviewed and was found to differ in significant ways from many other locales, for instance in favoring a process versus product distinction and not proceeding via a case-by-case approach, which in turn is increasing the likelihood of asynchronous approval processes and potential disruptions in international trade (Eriksson et al., 2019). Various jurisdictions are viewed as having more favorable regulatory processes for those seeking to commercialize GM, with the Philippines recently approving the cultivation of Golden Rice via its regulatory frameworks (Matacic, 2021) and Nigeria seen as a leader in Africa’s adoption of crop biotechnologies (Gbadegesin et al., 2022).
Australia has a dedicated regulatory system provided in the Commonwealth Gene Technology Act (2000) which was originally enacted to take the place of earlier voluntary regimes. The Gene Technology Act (2000) aimed to provide clear and transparent regulatory structures for GM research and commercial release, amidst growing public concerns including about whether the industry could be trusted to regulate itself. This regulatory structure is sometimes viewed as inefficient and overly burdensome by scientists and industry, but its activities and the transparency that they require should be embraced as an important part of fostering greater public engagement and fostering public trust by providing transparent and rigorous governmental oversight for GM research.
There is evidence that where communities are involved in co-production and inclusive dialog, there are more likely to be conditions that support an SLO to operate (e.g. Hall, 2014). What is particularly tricky in this context is that research has shown that if the impacts of science are focused only on economic and industrial benefits, there can be perceptions that the “social” aspects are being neglected. Hence scholars have argued that the SLO for science more generally should rely on co-construction with the public (for instance, research or funding priorities, or deployment of technologies or applications) rather than simply seeking public consent or capturing popular opinion (see Raman and Mohr, 2014), and that de-centering science in the co-production process can lead to better outcomes (Leith et al., 2014).
So, what should we do?
Based on best practices and what we know about public attitudes toward and engagement with gene editing and other novel biotechnologies to date, we recommend that scientific experts and science communicators should find ways to engage in genuine and meaningful dialog with various publics including those working in agriculture and community members at the earliest stages of research and on an ongoing basis. This call echoes other scholars’ arguments in the existing literature; for instance, the call for a "continuous discourse with society" (Pirscher and Theesfeld, 2018; Nature, 2021), with particular attention to values and use of participatory approaches (Bray and Ankeny, 2017; Bechtold, 2018)
Although space prohibits the presentation of a detailed model, key considerations are promoting consideration of their broader impacts including their likely risks and benefits, who stands to benefit and how, how their applications could fit within current and alternative agricultural regimes, their likely contributions to our food supply and our environment, and underlying broader values, rather than merely disseminating the technical details about these technologies and their potential economic and scientific benefits. In addition, transparency about various decisions associated with the development and deployment of these novel crops is critical, together with active engagement with and publicity of the associated regulatory processes and approvals.
The development of resilient crops is not only a scientific matter but is also associated with our society’s values and priorities, and our everyday lives. Publics reflecting together with scientists and others involved in this research and its products will allow consideration of underlying values associated with biotechnologies and their potential impacts and connections to even deeper societal goals such as responding to climate change and ensuring food security, including equal access to nourishing, culturally appropriate, sustainable, secure, and safe food, in part by forcing us to engage with and interrogate problems in our existing food system including our dominant production and consumption methods (Heldke, 2009). Participatory approaches permit the cultivation of more public awareness of alternatives without relying on vacuous approaches co-opted by industry or other interest groups, and without assuming that the publics simply denies or rejects science. As Van Eenennaam and Young (2018) note with reference to the use of gene editing in livestock, but clearly applicable in this context, public discussions around gene-edited food organisms will affect discussions about new technologies and food long into the future.
Future prospects and impact
The new genetic technologies described herein are developing at a rapid rate alongside advances in genomic selection and the targeted capturing and capitalizing on existing genetic diversity. The challenge and skill will be for traditional breeding programs to integrate the different tools with an amalgamated understanding of the physiological and cellular responses to abiotic stresses, considered not in isolation, but in the context of yield potential, agronomic traits, and the need for dynamic adaptations to suites of stresses. Our ability to deliver on the promise of the new genetic and field technologies will depend on discoveries that enable such future crops to be embedded in a more dynamic and responsive cropping system designed to respond to more variable and changing environments. While opportunities for improving climate resilience appear high, very few traits have been adopted commercially. Consequently, alongside technological development, a more inclusive dialog between researchers, industry, and society is essential if we are to deliver on the rapid advances needed to address food security.
Funding
All authors acknowledge the support of the Australian Research Council Training Centre for Future Crops Development (IC210100047); in addition, A.F.B. and B.J.P. were supported by an Australian Research Council Australian Laureate Fellowship (FL190100056).
Conflict of interest statement. None declared.
Contributor Information
Andrew F Bowerman, ARC Training Centre for Accelerated Future Crops Development, The Australian National University, Canberra, Australian Capital Territory, Australia.
Caitlin S Byrt, ARC Training Centre for Accelerated Future Crops Development, The Australian National University, Canberra, Australian Capital Territory, Australia.
Stuart John Roy, ARC Training Centre for Accelerated Future Crops Development, University of Adelaide, South Australia, Australia; School of Agriculture, Food and Wine & Waite Research Institute, University of Adelaide, Glen Osmond, South Australia, Australia.
Spencer M Whitney, ARC Training Centre for Accelerated Future Crops Development, The Australian National University, Canberra, Australian Capital Territory, Australia.
Jenny C Mortimer, ARC Training Centre for Accelerated Future Crops Development, University of Adelaide, South Australia, Australia; School of Agriculture, Food and Wine & Waite Research Institute, University of Adelaide, Glen Osmond, South Australia, Australia; Environmental Genomics and Systems Biology Division, Lawrence Berkeley National Laboratory, Berkeley, California 94720, USA.
Rachel A Ankeny, ARC Training Centre for Accelerated Future Crops Development, University of Adelaide, South Australia, Australia; School of Humanities, University of Adelaide, North Terrace, South Australia, Australia.
Matthew Gilliham, ARC Training Centre for Accelerated Future Crops Development, University of Adelaide, South Australia, Australia; School of Agriculture, Food and Wine & Waite Research Institute, University of Adelaide, Glen Osmond, South Australia, Australia.
Dabing Zhang, ARC Training Centre for Accelerated Future Crops Development, University of Adelaide, South Australia, Australia; School of Agriculture, Food and Wine & Waite Research Institute, University of Adelaide, Glen Osmond, South Australia, Australia.
Anthony A Millar, ARC Training Centre for Accelerated Future Crops Development, The Australian National University, Canberra, Australian Capital Territory, Australia.
Greg J Rebetzke, CSIRO Agriculture & Food, Canberra, Australian Capital Territory, Australia.
Barry J Pogson, ARC Training Centre for Accelerated Future Crops Development, The Australian National University, Canberra, Australian Capital Territory, Australia.
A.F.B. coordinated and led the writing and editing of the article; all authors contributed to writing and editing of the article.
The authors responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plcell) are: Andrew F. Bowerman (andrew.bowerman@anu.edu.au) and Barry J. Pogson (barry.pogson@anu.edu.au)
References
- Abdelraheem A, Thyssen GN, Fang DD, Jenkins JN, McCarty JC, Wedegaertner T, Zhang JF (2021) GWAS reveals consistent QTL for drought and salt tolerance in a MAGIC population of 550 lines derived from intermating of 11 Upland cotton (Gossypium hirsutum) parents. Mol Genet Genomics 296: 119–129 [DOI] [PubMed] [Google Scholar]
- Aguilar EA, Turner DW, Gibbs DJ, Armstrong W., Sivasithamparam K (2003) Oxygen distribution and movement, respiration and nutrient loading in banana roots (Musa spp. L.) subjected to aerated and oxygen-depleted environments. Plant Soil 253: 91–102 [Google Scholar]
- Ainsworth EA, Long SP (2021) 30 years of free‐air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation? Global Change Biol 27: 27–49 [DOI] [PubMed] [Google Scholar]
- Ali Z, Abul-faraj A, Li LX, Ghosh N, Piatek M, Mahjoub A, Aouida M, Piatek A, Baltes NJ, Voytas DF, et al. (2015) Efficient virus-mediated genome editing in plants using the CRISPR/Cas9 system. Mol Plant 8: 1288–1291 [DOI] [PubMed] [Google Scholar]
- Alley RB, Marotzke J, Nordhaus WD, Overpeck JT, Peteet DM, Pielke RA, Pierrehumbert RT, Rhines PB, Stocker TF, Talley LD, et al. (2003) Abrupt climate change. Science 299: 2005–2010 [DOI] [PubMed] [Google Scholar]
- Amani I,, Fischer RA,, Reynolds MP (1996) Canopy temperature depression association with yield of irrigated spring wheat cultivars in a hot slimate. J Agron Crop Sci 176: 119–129 [Google Scholar]
- An D, Chen J-G, Gao Y-Q, Li X, Chao Z-F, Chen Z-R, Li Q-Q, Han M-L, Wang Y-L, Wang Y-F, et al. (2017) AtHKT1 drives adaptation of Arabidopsis thaliana to salinity by reducing floral sodium content. PLoS Genet 13: e1007086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankeny RA, Bray HJ (2016) “If We're Happy to Eat It, Why Wouldn't We Be Happy to Give It to Our Children?” Articulating the complexities underlying women's ethical views on genetically modified food. Int J Fem Approaches Bioeth 9: 166–191 [Google Scholar]
- Asif MA, Garcia M, Tilbrook J, Brien C, Dowling K, Berger B, Schilling RK, Short L, Trittermann C, Gilliham M, et al. (2021) Identification of salt tolerance QTL in a wheat RIL mapping population using destructive and non-destructive phenotyping. Funct Plant Biol 48: 131–140 [DOI] [PubMed] [Google Scholar]
- Asif MA, Schilling RK, Tilbrook J, Brien C, Dowling K, Rabie H, Short L, Trittermann C, Garcia A, Barrett-Lennard EG, et al. (2018) Mapping of novel salt tolerance QTL in an Excalibur × Kukri doubled haploid wheat population. Theor Appl Genet 131: 2179–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseng S, Ewert F, Martre P, Rotter RP, Lobell DB, Cammarano D, Kimball BA, Ottman MJ, Wall GW, White JW, et al. (2015) Rising temperatures reduce global wheat production. Nat Clim Chang 5: 143–147 [Google Scholar]
- Atkin OK, Tjoelker MG (2003) Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci 8: 343–351 [DOI] [PubMed] [Google Scholar]
- Atlin GN, Frey KJ (1989) Breeding crop varieties for low-input agriculture. Am J Altern Agric 4: 53–58 [Google Scholar]
- Atlin GN, Cairns JE, Das B (2017) Rapid breeding and varietal replacement are critical to adaptation of cropping systems in the developing world to climate change. Glob Food Secur-Agric Policy 12: 31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atucha-Zamkova AA, Steele KA, Smith AR (2021) Modelling the impact of climate change on the occurrence of frost damage in Sitka spruce (Picea sitchensis) in Great Britain. Forestry 94: 664–676 [Google Scholar]
- Babgohari MZ, Niazi A, Moghadam AA, Deihimi T, Ebrahimie E (2013) Genome-wide analysis of key salinity-tolerance transporter (HKT1;5) in wheat and wild wheat relatives (A and D genomes). In Vitro Cell Dev Biol-Plant 49: 97–106 [Google Scholar]
- Barlow KM, Christy BP, O'Leary GJ, Riffkin PA, Nuttall JG (2015) Simulating the impact of extreme heat and frost events on wheat crop production: A review. Field Crops Res 171: 109–119 [Google Scholar]
- Barnard RL, Osborne CA, Firestone MK (2013) Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J 7: 2229–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrero JM, Porfirio L, Hughes T, Chen J, Dillon S, Gubler F, Ral JPF (2020) Evaluation of the impact of heat on wheat dormancy, late maturity alpha-amylase and grain size under controlled conditions in diverse germplasm. Sci Rep 10: 17800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkowski B,, Baum CM (2019) Dealing with rejection: An application of the exit-voice framework to genome-edited food. Front Bioeng Biotechnol 7: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkowski B, Theesfeld I, Pirscher F, Timaeus J (2018) Snipping around for food: economic, ethical and policy implications of CRISPR/Cas genome editing. Geoforum 96: 172–180 [Google Scholar]
- Baxter I, Brazelton JN, Yu D, Huang YS, Lahner B, Yakubova E, Li Y, Bergelson J, Borevitz JO, Nordborg M, et al. (2010) A coastal cline in sodium accumulation in Arabidopsis thaliana is driven by natural variation of the sodium transporter AtHKT1;1. PLoS Genet 6:e1001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold S (2018) Beyond risk considerations: Where and how can a debate about non-safety related issues of genome editing in agriculture take place? Front Plant Sci 9: 1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck HE, Wood EF, Pan M, Fisher CK, Miralles DG, Van Dijk AIJM, McVicar TR, Adler RF (2019) MSWEP V2 global 3-hourly 0.1° precipitation: methodology and quantitative assessment. Bull Am Meteorol Soc 100: 473–500 [Google Scholar]
- Bloom AJ, Plant RE (2021) Wheat grain yield decreased over the past 35 years, but protein content did not change. J Exp Bot 72: 6811–6821 [DOI] [PubMed] [Google Scholar]
- BOM. (2022) La Nina event declared-above average rainfall likely for eastern Australia (https://media.bom.gov.au/releases/1069/la-nina-event-declared-above-average-rainfall-likely-for-eastern-australia/: Australian Beareau of Meteorology) (September 22, 2022)
- Borjigin C, Schilling RK, Jewell N, Brien C, Sanchez-Ferrero JC, Eckermann PJ, Watson-Haigh NS, Berger B, Pearson AS, Roy SJ (2021) Identifying the genetic control of salinity tolerance in the bread wheat landrace Mocho de Espiga Branca. Funct Plant Biol 48: 1148–1160 [DOI] [PubMed] [Google Scholar]
- Borjigin C, Schilling RK, Bose J, Hrmova M, Qiu J, Wege S, Situmorang A, Byrt C, Brien C, Berger B, et al. (2020) A single nucleotide substitution in TaHKT1;5‐D controls shoot Na+ accumulation in bread wheat. Plant, Cell Environ 43: 2158–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton CA, Ritchie PDL, Lenton TM (2020) Abrupt changes in Great Britain vegetation carbon projected under climate change. Global Change Biol 26: 4436–4448 [DOI] [PubMed] [Google Scholar]
- Bouma TJ, Visser R, Janssen JHJA, Kock MJ, Leeuwen PH, Lambers H (1994) Respiratory energy requirements and rate of protein turnover in vivo determined by the use of an inhibitor of protein synthesis and a probe to assess its effect. Physiol Plant 92: 585–594 [Google Scholar]
- Brás TA, Seixas J, Carvalhais N, Jägermeyr J (2021) Severity of drought and heatwave crop losses tripled over the last five decades in Europe. Environ Res Lett 16: 065012 [Google Scholar]
- Bray HJ, Ankeny RA (2015) What do food labels teach people about food ethics? InFlowers R and Swan E, eds, Food Pedagogies. Ashgate, London, pp 185–200 [Google Scholar]
- Bray HJ, Ankeny RA (2017) Not just about "the science": science education and attitudes to genetically modified foods among women in Australia. New Genet Soc 36: 1–21 [Google Scholar]
- Brossard D, Nisbet MC (2007) Deference to scientific authority among a low information public: understanding US Opinion on agricultural biotechnology. Int J Public Opin Res 19: 24–52 [Google Scholar]
- Bruckner PL,, Frohberg RC (1987) Rate and duration of grain fill in spring wheat 1. Crop Sci 27: 451–455 [Google Scholar]
- Bush DR (2020) Identifying the pathways that control resource allocation in higher plants. Proc Natl Acad Sci USA 117: 8669–8671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busoms S, Paajanen P, Marburger S, Bray S, Huang XY, Poschenrieder C, Yant L, Salt DE (2018) Fluctuating selection on migrant adaptive sodium transporter alleles in coastal Arabidopsis thaliana. Proc Natl Acad Sci USA 115: E12443–E12452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrt CS, Platten JD, Spielmeyer W, James RA, Lagudah ES, Dennis ES, Tester M, Munns R (2007) HKT1;5-like cation transporters linked to Na+ exclusion loci in wheat, Nax2 and Kna1. Plant Physiol 143: 1918–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrt CS, Xu B, Krishnan M, Lightfoot DJ, Athman A, Jacobs AK, Watson-Haigh NS, Plett D, Munns R, Tester M, et al. (2014) The Na+ transporter, TaHKT1;5-D, limits shoot Na+ accumulation in bread wheat. Plant J 80: 516–526 [DOI] [PubMed] [Google Scholar]
- Cabrera M, Boronat S, Marte L, Vega M, Pérez P, Ayté J, Hidalgo E (2020) Chaperone-facilitated aggregation of thermo-sensitive proteins shields them from degradation during heat stress. Cell Reports 30: 2430–2443.e2434 [DOI] [PubMed] [Google Scholar]
- Canto T, Aranda MA, Fereres A (2009) Climate change effects on physiology and population processes of hosts and vectors that influence the spread of hemipteran-borne plant viruses. Global Change Biol 15: 1884–1894 [Google Scholar]
- Casal JJ, , Balasubramanian S (2019) Thermomorphogenesis. Annu Rev Plant Biol 70: 321–346 [DOI] [PubMed] [Google Scholar]
- Causier B, Hopes T, McKay M, Paling Z, Davies B (2022) Plants utilise ancient conserved peptide upstream open reading frames in stress-responsive translational regulation. Plant Cell Environ 45: 1229–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli S, Grando S (2020) Evolutionary plant breeding as a response to the complexity of climate change. iScience 23: 101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KX, Phua SY, Crisp P, McQuinn R, Pogson BJ (2016a) Learning the languages of the chloroplast: retrograde signaling and beyond. Ann Rev Plant Biol 67: 25–53 [DOI] [PubMed] [Google Scholar]
- Chan KX, Mabbitt PD, Phua SY, Mueller JW, Nisar N, Gigolashvili T, Stroeher E, Grassl J, Arlt W, Estavillo GM, et al. (2016b) Sensing and signaling of oxidative stress in chloroplasts by inactivation of the SAL1 phosphoadenosine phosphatase. Proc Natl Acad Sci USA 113: E4567–E4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Stein ML, Wang J, Kotamarthi VR, Moyer EJ (2016) Changes in spatiotemporal precipitation patterns in changing climate conditions. J Climate 29: 8355–8376 [Google Scholar]
- Chapman SC, Chakraborty S, Dreccer MF, Howden SM (2012) Plant adaptation to climate change—opportunities and priorities in breeding. Crop Pasture Sci 63: 251–268, 218 [Google Scholar]
- Chen J, Li S, He Y, Li J, Xia L (2022) An update on precision genome editing by homology-directed repair in plants. Plant Physiol 188: 1780–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K,, Wang Y,, Zhang R,, Zhang H,, Gao C (2019) CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu Rev Plant Biol 70: 667–697 [DOI] [PubMed] [Google Scholar]
- Chen Q, Liu R, Wu Y, Wei S, Wang Q, Zheng Y, Xia R, Shang X, Yu F, Yang X, et al. (2021) ERAD-related E2 and E3 enzymes modulate the drought response by regulating the stability of PIP2 aquaporins. Plant Cell 33: 2883–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Shi L, Chen YQ, Zhu L, Zhang DS, Xiao S, Aharoni A, Shi JX, Xu J (2019b) Arabidopsis HSP70-16 is required for flower opening under normal or mild heat stress temperatures. Plant Cell Environ 42: 1190–1204 [DOI] [PubMed] [Google Scholar]
- Cheng WG, Sakai H, Yagi K, Hasegawa T (2009) Interactions of elevated CO2 and night temperature on rice growth and yield. Agr Forest Meteorol 149: 51–58 [Google Scholar]
- Chennakesavulu K, Singh H, Trivedi PK, Jain M, Yadav SR (2022) State-of-the-art in CRISPR technology and engineering drought, salinity, and thermo-tolerant crop plants. Plant Cell Reports 41: 815–831 [DOI] [PubMed] [Google Scholar]
- Chenu K, van Oosterom EJ, McLean G, Deifel KS, Fletcher A, Geetika G, Tirfessa A, Mace ES, Jordan DR, Sulman R, et al. (2018) Integrating modelling and phenotyping approaches to identify and screen complex traits: transpiration efficiency in cereals. J Exp Bot 69: 3181–3194 [DOI] [PubMed] [Google Scholar]
- Christmann A, Grill E, Huang J (2013) Hydraulic signals in long-distance signaling. Curr Opin Plant Biol 16: 293–300 [DOI] [PubMed] [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD (2004) Breeding for high water-use efficiency. J Exp Bot 55: 2447–2460 [DOI] [PubMed] [Google Scholar]
- Cooper M, Messina CD (2023) Breeding crops for drought-affected environments and improved climate resilience. Plant Cell 35: 162–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M, Voss-Fels KP, Messina CD, Tang T, Hammer GL (2021) Tackling G x E x M interactions to close on-farm yield-gaps: creating novel pathways for crop improvement by predicting contributions of genetics and management to crop productivity. Theor Appl Genet 134: 1625–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormick C, Mercer R (2017) Community Attitudes to Gene Technology. Instinct and Reason, Sydney [Google Scholar]
- Corwin DL (2021) Climate change impacts on soil salinity in agricultural areas. Eur J Soil Sci 72: 842–862 [Google Scholar]
- Cotrufo MF, Ineson P, Scott A (1998) Elevated CO 2 reduces the nitrogen concentration of plant tissues. Global Change Biol 4: 43–54 [Google Scholar]
- Crespo-Herrera LA, Crossa J, Huerta-Espino J, Mondal S, Velu G, Juliana P, Vargas M, Pérez-Rodríguez P, Joshi AK, Braun HJ, et al. (2021) Target population of environments for wheat breeding in India: definition, prediction and genetic gains. Front Plant Sci 12: 638520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimp SJ, Zheng B, Khimashia N, Gobbett DL, Chapman S, Howden M, Nicholls N (2016) Recent changes in southern Australian frost occurrence: implications for wheat production risk. Crop Pasture Sci 67: 801–811 [Google Scholar]
- Dalakouras A, Wassenegger M, McMillan JN, Cardoza V, Maegele I, Dadami E, Runne M, Krczal G, Wassenegger M (2016) Induction of silencing in plants by high-pressure spraying of in vitro-synthesized small RNAs. Front Plant Sci 7: 1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszkowska-Golec A (2016) The role of abscisic acid in drought stress: how ABA helps plants to cope with drought stress. InHossain MA, Wani SH, Bhattacharjee S, Burritt DJ, Tran L-SP, eds, Springer International Publishing, Cham, pp 123–151 [Google Scholar]
- Debucquet G,, Baron R,, Cardinal M (2020) Lay and scientific categorizations of new breeding techniques: Implications for food policy and genetically modified organism legislation. Public Underst Sci 29: 524–543 [DOI] [PubMed] [Google Scholar]
- Deckers J (2005) Are scientists right and non-scientists wrong? Reflections on discussions of GM. J Agric Environ Ethics 18: 451–478 [Google Scholar]
- Demirer GS, Silva TN, Jackson CT, Thomas JB, Ehrhardt DW, Rhee SY, Mortimer JC, Landry MP (2021) Nanotechnology to advance CRISPR-Cas genetic engineering of plants. Nat Nanotechnol 16: 243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempewolf H, Eastwood RJ, Guarino L, Khoury CK, Muller JV, Toll J (2014) Adapting agriculture to climate change: a global initiative to collect, conserve, and use crop wild relatives. Agroecol Sustain Food Syst 38: 369–377 [Google Scholar]
- Dier M, Sickora J, Erbs M, Weigel HJ, Zorb C, Manderscheid R (2019) Positive effects of free air CO2 enrichment on N remobilization and post-anthesis N uptake in winter wheat. Field Crops Res 234: 107–118 [Google Scholar]
- Ding Y, Yang S (2022) Surviving and thriving: how plants perceive and respond to temperature stress. Dev Cell 57: 947–958 [DOI] [PubMed] [Google Scholar]
- Ding YL, Shi YT, Yang SH (2020) Molecular regulation of plant responses to environmental temperatures. Mol Plant 13: 544–564 [DOI] [PubMed] [Google Scholar]
- Dubey A, Malla MA, Khan F, Chowdhary K, Yadav S, Kumar A, Sharma S, Khare PK, Khan ML (2019) Soil microbiome: a key player for conservation of soil health under changing climate. Biodivers Conserv 28: 2405–2429 [Google Scholar]
- Ellison EE, Nagalakshmi U, Gamo ME, Huang PJ, Dinesh-Kumar S, Voytas DF (2020) Multiplexed heritable gene editing using RNA viruses and mobile single guide RNAs. Nat Plants 6: 620–624 [DOI] [PubMed] [Google Scholar]
- Eriksson D, Kershen D, Nepomuceno A, Pogson BJ, Prieto H, Purnhagen K, Smyth S, Wesseler J, Whelan A (2019) A comparison of the EU regulatory approach to directed mutagenesis with that of other jurisdictions, consequences for international trade and potential steps forward. New Phytol 222: 1673–1684. [DOI] [PubMed] [Google Scholar]
- Ermakova M, Osborn H, Groszmann M, Bala S, Bowerman A, McGaughey S, Byrt C, Alonso-Cantabrana H, Tyerman S, Furbank RT, et al. (2021) Expression of a CO2-permeable aquaporin enhances mesophyll conductance in the C4 species Setaria viridis. eLife 10: e70095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estavillo GM, Crisp PA, Pornsiriwong W, Wirtz M, Collinge D, Carrie C, Giraud E, Whelan J, David P, Javot H, et al. (2011) Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell 23: 3992–4012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR (2013) Improving photosynthesis. Plant Physiol 162: 1780–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan XR, Jiang HZ, Meng LJ, Chen JG (2021) Gene mapping, cloning and association analysis for salt tolerance in rice. Int J Mol Sci 22: 11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar K, Bryant D, Cope‐Selby N (2014) Understanding and engineering beneficial plant–microbe interactions: plant growth promotion in energy crops. Plant Biotechnol J 12: 1193–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari L (2022) Farmers' attitude toward CRISPR/Cas9: the case of blast resistant rice. Agribusiness 38: 175–194 [Google Scholar]
- Flohr BM, Ouzman J, McBeath TM, Rebetzke GJ, Kirkegaard JA, Llewellyn RS (2021) Redefining the link between rainfall and crop establishment in dryland cropping systems. Agr Syst 190: 103105 [Google Scholar]
- Gabilly ST, Baker CR, Wakao S, Crisanto T, Guan K, Bi K, Guiet E, Guadagno CR, Niyogi KK (2019) Regulation of photoprotection gene expression in Chlamydomonas by a putative E3 ubiquitin ligase complex and a homolog of CONSTANS. Proc Natl Acad Sci USA 116: 17556–17562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Abellan JO, Egea I, Pineda B, Sanchez-Bel P, Belver A, Garcia-Sogo B, Flores FB, Atares A, Moreno V, Bolarin MC (2014) Heterologous expression of the yeast HAL5 gene in tomato enhances salt tolerance by reducing shoot Na+ accumulation in the long term. Physiol Plant 152: 700–713 [DOI] [PubMed] [Google Scholar]
- Gatica-Arias A, Valdez-Melara M, Arrieta-Espinoza G, Albertazzi-Castro FJ, Madrigal-Pana J (2019) Consumer attitudes toward food crops developed by CRISPR/Cas9 in Costa Rica. Plant Cell, Tissue Organ Culture 139: 417–427 [Google Scholar]
- Gbadegesin LA, Ayeni EA, Tettey CK, Uyanga VA, Aluko OO, Ahiakpa JK, Okoye CO, Mbadianya JI, Adekoya MA, Aminu RO, et al. (2022) GMOs in Africa: status, adoption and public acceptance. Food Control 141: 109193. [Google Scholar]
- Genc Y,, Taylor J,, Lyons G,, Li Y,, Cheong J,, Appelbee M,, Oldach K,, Sutton T (2019) Bread wheat with high salinity and sodicity tolerance. Front Plant Sci 10: 1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez VD, Miralles DJ, Garcia GA, Serrago RA (2021) Can crop management reduce the negative effects of warm nights on wheat yield? Field Crops Res 261: 108010 [Google Scholar]
- Graham NE (1994) Decadal-scale climate variability in the tropical and North Pacific during the 1970s and 1980s: observations and model results. Clim Dyn 10: 135–162 [Google Scholar]
- Grant W,, Bray H,, Harms R,, Ankeny R,, Leach J (2021) Consumer responses to the use of NBTs in the production of food: A systematic literature review. The Australian National University, Canberra, Australia [Google Scholar]
- GRDC (2018) Hay and Silage Fact Sheet: Making the Most of a Failed Winter Crop Grains Research and Development Corporation, Canberra, Australia [Google Scholar]
- Gregory J, Miller S (1998) Science in Public: Communication, Culture, and Credibility. Plenum Trade, New York [Google Scholar]
- Guan QM, Lu XY, Zeng HT, Zhang YY, Zhu JH (2013) Heat stress induction of miR398 triggers a regulatory loop that is critical for thermotolerance in Arabidopsis. Plant J 74: 840–851 [DOI] [PubMed] [Google Scholar]
- Guan R, Qu Y, Guo Y, Yu L, Liu Y, Jiang J, Chen J, Ren Y, Liu G, Tian L, et al. (2014) Salinity tolerance in soybean is modulated by natural variation in GmSALT3. Plant J 80: 937–950 [DOI] [PubMed] [Google Scholar]
- Guo M, Rupe MA, Wei J, Winkler C, Goncalves-Butruille M, Weers BP, Cerwick SF, Dieter JA, Duncan KE, Howard RJ, et al. (2014) Maize ARGOS1 (ZAR1) transgenic alleles increase hybrid maize yield. J Exp Bot 65: 249–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall NL (2014) Can the "social licence to operate" concept enhance engagement and increase acceptance of renewable energy? A case study of wind farms in Australia. Soc Epistemol 28: 219–238 [Google Scholar]
- Hammer GL, van Oosterom E, McLean G, Chapman SC, Broad I, Harland P, Muchow RC (2010) Adapting APSIM to model the physiology and genetics of complex adaptive traits in field crops. J Exp Bot 61: 2185–2202 [DOI] [PubMed] [Google Scholar]
- Harris J (2015) Abscisic acid: hidden architect of root system structure. Plants 4: 548–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart PS, Nisbet EC (2012) Boomerang effects in science communication: how motivated reasoning and identity cues amplify opinion polarization about climate mitigation policies. Commun Res 39: 701–723 [Google Scholar]
- Hartmann DL, Klein Tank A.M.G., Rusticucci M, Alexander LV, Broennimann S, Charabi Y, Dentener FJ, Dlugokencky EJ, Easterling DR, Kaplan A, et al. (2013) Observations: atmosphere and surface. InStocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgle PM, eds, Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK and New York, NY, USA, pp 159–254 [Google Scholar]
- Hauben M, Haesendonckx B, Standaert E, Van Der Kelen K, Azmi A, Akpo H, Van Breusegem F, Guisez Y, Bots M, Lambert B, et al. (2009) Energy use efficiency is characterized by an epigenetic component that can be directed through artificial selection to increase yield. Proc Natl Acad Sci USA 106: 20109–20114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayta S, Smedley MA, Demir SU, Blundell R, Hinchliffe A, Atkinson N, Harwood WA (2019) An efficient and reproducible Agrobacterium-mediated transformation method for hexaploid wheat (Triticum aestivum L.). Plant Methods 15: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Fang JY, Xu W, Shi PJ (2022) Substantial increase of compound droughts and heatwaves in wheat growing seasons worldwide. Int J Climatol 42: 5038–5054 [Google Scholar]
- Hegerl GC, Brönnimann S, Schurer A, Cowan T (2018) The early 20th century warming: Anomalies, causes, and consequences. WIREs Climate Change 9: e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heichel GH (1971) Confirming measurements of respiration and photosynthesis with dry matter accumulation. Photosynthetica 5: 93–98 [Google Scholar]
- Heldke LM (2009) Food security: three conceptions of access—charity, rights, and co-responsibility. InWalter L, ed, Society, Culture, and Ethics, Praeger, Santa Barbara, USA, pp 213–225 [Google Scholar]
- Hendel E, Bacher H, Oksenberg A, Walia H, Schwartz N, Peleg Z (2021) Deciphering the genetic basis of wheat seminal root anatomy uncovers ancestral axial conductance alleles. Plant, Cell Environ 44: 1921–1934 [DOI] [PubMed] [Google Scholar]
- Henderson SW, Dunlevy JD, Wu Y, Blackmore DH, Walker RR, Edwards EJ, Gilliham M, Walker AR (2018) Functional differences in transport properties of natural HKT1;1 variants influence shoot Na+ exclusion in grapevine rootstocks. New Phytol 217: 1113–1127 [DOI] [PubMed] [Google Scholar]
- Hendriks P-W, Gurusinghe S, Ryan PR, Rebetzke GJ, Weston LA (2022a) Competitiveness of early vigour wheat (Triticum aestivum L.) genotypes is established at early growth stages. Agronomy 12: 377 [Google Scholar]
- Hendriks PW, Ryan PR, Hands P, Rolland V, Gurusinghe S, Weston LA, Rebetzke GJ, Delhaize E (2022b) Selection for early shoot vigour in wheat increases root hair length but reduces epidermal cell size of roots and leaves. J Exp Bot 73: 2499–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman Z, Gobbett DL, Horan H (2017) Climate trends account for stalled wheat yields in Australia since 1990. Global Change Biol 23: 2071–2081 [DOI] [PubMed] [Google Scholar]
- Holzworth DP, Huth NI, Devoil PG, Zurcher EJ, Herrmann NI, McLean G, Chenu K, van Oosterom EJ, Snow V, Murphy C, et al. (2014) APSIM - Evolution towards a new generation of agricultural systems simulation. Environ Modell Softw 62: 327–350 [Google Scholar]
- Houshmandfar A, Ota N, O'Leary GJ, Zheng B, Chen Y, Tausz‐Posch S, Fitzgerald GJ, Richards R, Rebetzke GJ, Tausz M (2020) A reduced‐tillering trait shows small but important yield gains in dryland wheat production. Global Change Biol 26: 4056–4067 [DOI] [PubMed] [Google Scholar]
- Houston K, Qiu J, Wege S, Hrmova M, Oakey H, Qu Y, Smith P, Situmorang A, Macaulay M, Flis P, et al. (2020) Barley sodium content is regulated by natural variants of the Na+ transporter HvHKT1;5. Commun Biol 3: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Kuang L, Wu L, Shen Q, Han Y, Jiang L, Wu D, Zhang G (2020) The HKT transporter HvHKT1;5 negatively regulates salt tolerance. Plant Physiol 182: 584–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueso S, García C, Hernández T (2012) Severe drought conditions modify the microbial community structure, size and activity in amended and unamended soils. Soil Biol Biochem 50: 167–173 [Google Scholar]
- Hughes N, Galeano D, Hattfield-Dodds S (2019) The effects of drought and climate variability on Australian farms. Australian Bureau of Agricultural and Resource Economics and Sciences, Canberra, Australia. https://doi.org/10.25814/5de84714f6e08 [Google Scholar]
- Hunt JR, Hayman PT, Richards RA, Passioura JB (2018) Opportunities to reduce heat damage in rain-fed wheat crops based on plant breeding and agronomic management. Field Crops Res 224: 126–138 [Google Scholar]
- Hussain S, Gomes MM, Yano K, Nambara E (2019) Chapter Seven - Interactions between abscisic acid and other hormones. InSeo M, Marion-Poll A, eds, Advances in Botanical Research, Academic Press, London, UK, pp 255–280 [Google Scholar]
- IPBES (2018) The IPBES assessment report on land degradation and restoration. InMontanarella L, Scholes R, Brainich A, eds, Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, Bonn, Germany, p 774 [Google Scholar]
- IPCC (2019) InShukla PR, Skea J, Buendia EC, Masson-Delmotte V, Pörtner H-O, Roberts DC, Zhai P, Slade R, Connors S, Diemen RV, et al. , eds, Climate Change and Land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems, The Intergovernmental Panel on Climate Change, Paris [Google Scholar]
- IPCC (2022) Climate change 2022: impacts, adaptation, and vulnerability. InPörtner H-O, Roberts DC, Tignor M, Poloczanska ES, Mintenbeck K, Alegría A, Craig M, Langsdorf S, Löschke S, Möller V, et al. , eds, Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, UK and New York, NY, USA [Google Scholar]
- Jablonski LM, Wang XZ, Curtis PS (2002) Plant reproduction under elevated CO2 conditions: a meta-analysis of reports on 79 crop and wild species. New Phytol 156: 9–26 [Google Scholar]
- James RA, Davenport RJ, Munns R (2006) Physiological characterization of two genes for Na+ exclusion in durum wheat, Nax1 and Nax2. Plant Physiol 142: 1537–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James RA, Blake C, Zwart AB, Hare RA, Rathjen AJ, Munns R (2012) Impact of ancestral wheat sodium exclusion genes Nax1 and Nax2 on grain yield of durum wheat on saline soils. Funct Plant Biol 39: 609–618 [DOI] [PubMed] [Google Scholar]
- Jansson JK, Hofmockel KS (2020) Soil microbiomes and climate change. Nat Rev Microbiol 18: 35–46 [DOI] [PubMed] [Google Scholar]
- Jaynes DB, Colvin TS, Karlen DL, Cambardella CA, Meek DW (2001) Nitrate loss in subsurface drainage as affected by nitrogen fertilizer rate. J Environ Qual 30: 1305–1314 [DOI] [PubMed] [Google Scholar]
- Jin S, Zong Y, Gao Q, Zhu ZX, Wang YP, Qin P, Liang CZ, Wang DW, Qiu JL, Zhang F, et al. (2019) Cytosine, but not adenine, base editors induce genome-wide off-target mutations in rice. Science 364: 292–295 [DOI] [PubMed] [Google Scholar]
- Jung JH, Domijan M, Klose C, Biswas S, Ezer D, Gao MJ, Khattak AK, Box MS, Charoensawan V, Cortijo S, et al. (2016) Phytochromes function as thermosensors in Arabidopsis. Science 354: 886–889 [DOI] [PubMed] [Google Scholar]
- Juroszek P, von Tiedemann A (2013) Climate change and potential future risks through wheat diseases: a review. Eur J Plant Pathol 136: 21–33 [Google Scholar]
- Kang BC, Yun JY, Kim ST, Shin Y, Ryu J, Choi M, Woo JW, Kim JS (2018) Precision genome engineering through adenine base editing in plants. Nat Plants 4: 427–431 [DOI] [PubMed] [Google Scholar]
- Kato-Nitta N, Maeda T, Inagaki Y, Tachikawa M (2019) Expert and public perceptions of gene-edited crops: attitude changes in relation to scientific knowledge. Palgrave Commun 5: 137 [Google Scholar]
- Khorasani GR, Jedel PE, Helm JH, Kennelly JJ (1997) Influence of stage of maturity on yield components and chemical composition of cereal grain silages. Can J Anim Sci 77: 259–267 [Google Scholar]
- Kobayashi NI, Yamaji N, Yamamoto H, Okubo K, Ueno H, Costa A, Tanoi K, Matsumura H, Fujii-Kashino M, Horiuchi T, et al. (2017) OsHKT1;5 mediates Na+ exclusion in the vasculature to protect leaf blades and reproductive tissues from salt toxicity in rice. Plant J 91: 657–670 [DOI] [PubMed] [Google Scholar]
- Koch A, Biedenkopf D, Furch A, Weber L, Rossbach O, Abdellatef E, Linicus L, Johannsmeier J, Jelonek L, Goesmann A, et al. (2016) An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog e1005901, 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR (2016) Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533: 420–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong JX, Martin-Ortigosa S, Finer J, Orchard N, Gunadi A, Batts LA, Thakare D, Rush B, Schmitz O, Stuiver M, et al. (2020) Overexpression of the transcription factor growth-regulating factor5 improves transformation of dicot and monocot species. Front Plant Sci 11: 572319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibich H, Van Loon AF, Schroter K, Ward PJ, Mazzoleni M, Sairam N, Abeshu GW, Agafonova S, AghaKouchak A, Aksoy H, et al. (2022) The challenge of unprecedented floods and droughts in risk management. Nature 608: 80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromdijk J, Głowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP (2016) Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354: 857–861 [DOI] [PubMed] [Google Scholar]
- Lamichhane JR (2021) Rising risks of late-spring frosts in a changing climate. Nat Clim Change 11: 554–555 [Google Scholar]
- Lau JA, Lennon JT (2011) Evolutionary ecology of plant-microbe interactions: soil microbial structure alters selection on plant traits. New Phytol 192: 215–224 [DOI] [PubMed] [Google Scholar]
- Lea E (2005) Beliefs about genetically modified foods: a qualitative and quantitative exploration. Ecol Food Nutr 44: 437–454 [Google Scholar]
- Lee U, Rioflorido I, Hong SW, Larkindale J, Waters ER, Vierling E (2007) The Arabidopsis ClpB/Hsp100 family of proteins: chaperones for stress and chloroplast development. Plant J 49: 115–127 [DOI] [PubMed] [Google Scholar]
- Leith P, Ogier E, Haward M (2014) Science and social license: defining environmental sustainability of Atlantic salmon aquaculture in South-Eastern Tasmania, Australia. Soc Epistemol 28: 277–296 [Google Scholar]
- Leng GY, Hall J (2019) Crop yield sensitivity of global major agricultural countries to droughts and the projected changes in the future. Sci Total Environ 654: 811–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonelli L, Brooks MD, Niyogi KK (2017) Engineering the lutein epoxide cycle into Arabidopsis thaliana. Proc Natl Acad Sci USA 114: E7002–E7008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C,, Zhang R,, Meng X,, Chen S,, Zong Y,, Lu C,, Qiu J-L,, Chen Y-H,, Li J,, Gao C (2020) Targeted, random mutagenesis of plant genes with dual cytosine and adenine base editors. Nat Biotechnol 38: 875–882 [DOI] [PubMed] [Google Scholar]
- Li G, Kuijer HNJ, Yang XJ, Liu HR, Shen CQ, Shi J, Betts N, Tucker MR, Liang WQ, Waugh R, et al. (2021) MADS1 maintains barley spike morphology at high ambient temperatures. Nat Plants 7: 1093–1107 [DOI] [PubMed] [Google Scholar]
- Li X, Ingvordsen CH, Weiss M, Rebetzke GJ, Condon AG, James RA, Richards RA (2019) Deeper roots associated with cooler canopies, higher normalized difference vegetation index, and greater yield in three wheat populations grown on stored soil water. J Exp Bot 70: 4963–4974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Chen KL, Li TD, Zhang Y, Wang YP, Zhao Q, Liu JX, Zhang HW, Liu CM, Ran YD, et al. (2017) Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat Commun 8: 14261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MY, Chai KH, Ko SS, Kuang LY, Lur HS, Charng YY (2014) A positive feedback loop between HEAT SHOCK PROTEIN101 and HEAT STRESS-ASSOCIATED 32-KD PROTEIN modulates long-term acquired thermotolerance illustrating diverse heat stress responses in rice varieties1 W OPEN. Plant Physiol 164: 2045–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Zong Y, Xue C, Wang S, Jin S, Zhu Z, Wang Y, Anzalone AV, Raguram A, Doman JL, et al. (2020) Prime genome editing in rice and wheat. Nat Biotechnol 38: 582–585 [DOI] [PubMed] [Google Scholar]
- Liu B, Martre P, Ewert F, Porter JR, Challinor AJ, Mueller C, Ruane AC, Waha K, Thorburn PJ, Aggarwal PK, et al. (2019) Global wheat production with 1.5 and 2.0 degrees C above pre-industrial warming. Global Change Biol 25: 1428–1444 [DOI] [PubMed] [Google Scholar]
- Liu L, Gallagher J, Arevalo ED, Chen R, Skopelitis T, Wu QY, Bartlett M, Jacksont D (2021) Enhancing grain-yield-related traits by CRISPR-Cas9 promoter editing of maize CLE genes. Nat Plants 7: 287–294 [DOI] [PubMed] [Google Scholar]
- Lockie S, Lawrence G, Lyons K, Grice J (2005) Factors underlying support or opposition to biotechnology among Australian food consumers and implications for retailer-led food regulation. Food Policy 30: 399–418 [Google Scholar]
- Long SP, Marshall-Colon A, Zhu XG (2015) Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 161: 56–66 [DOI] [PubMed] [Google Scholar]
- Lopes MS, El-Basyoni I, Baenziger PS, Singh S, Royo C, Ozbek K, Aktas H, Ozer E, Ozdemir F, Manickavelu A, et al. (2015) Exploiting genetic diversity from landraces in wheat breeding for adaptation to climate change. J Exp Bot 66: 3477–3486 [DOI] [PubMed] [Google Scholar]
- López-Castañeda C, Richards RA (1994) Variation in temperate cereals in rain-fed environments .1. Grain-yield, biomass and agronomic characteristics. Field Crops Res 37: 51–62 [Google Scholar]
- López-Castañeda C, Richards RA, Farquhar GD, Williamson RE (1996) Seed and seedling characteristics contributing to variation in early vigor among temperate cereals. Crop Sci 36: 1257–1266 [Google Scholar]
- Lowe K, Wu E, Wang N, Hoerster G, Hastings C, Cho MJ, Scelonge C, Lenderts B, Chamberlin M, Cushatt J, et al. (2016) Morphogenic regulators baby boom and wuschel improve monocot transformation . Plant Cell 28: 1998–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo MJ, Zhang YX, Chen K, Kong MS, Song W, Lu BS, Shi YX, Zhao YX, Zhao JR (2019) Mapping of quantitative trait loci for seedling salt tolerance in maize. Mol Breed 39: 64 [Google Scholar]
- Lychuk TE, Moulin AP, Lemke RL, Izaurralde RC, Johnson EN, Olfert OO, Brandt SA (2021) Modelling the effects of climate change, agricultural inputs, cropping diversity, and environment on soil nitrogen and phosphorus: a case study in Saskatchewan, Canada. Agric Water Manag 252: 106850 [Google Scholar]
- Lynch JP (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Botany 112: 347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher MF, Nasti RA, Vollbrecht M, Starker CG, Clark MD, Voytas DF (2020) Plant gene editing through de novo induction of meristems. Nat Biotechnol 38: 84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner P,, Crowley D,, Yang CH (2004) Development of specific rhizosphere bacterial communities in relation to plant species, nutrition and soil type. Plant Soil 261: 199–208 [Google Scholar]
- Matacic C (2021) Golden rice to sprout in the Philippines. Science 373: 472–47234326212 [Google Scholar]
- McFadden BR, Smyth SJ (2019) Perceptions of genetically engineered technology in developed areas. Trends Biotechnol 37: 447–451 [DOI] [PubMed] [Google Scholar]
- Mian A, Oomen R, Isayenkov S, Sentenac H, Maathuis FJM, Very AA (2011) Over-expression of an Na+- and K+-permeable HKT transporter in barley improves salt tolerance. Plant J 68: 468–479 [DOI] [PubMed] [Google Scholar]
- Mitter N, Worrall EA, Robinson KE, Li P, Jain RG, Taochy C, Fletcher SJ, Carroll BJ, Lu GQ, Xu ZP (2017) Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat Plants 3: 16207. [DOI] [PubMed] [Google Scholar]
- Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11: 15–19 [DOI] [PubMed] [Google Scholar]
- Molla KA, Sretenovic S, Bansal KC, Qi YP (2021) Precise plant genome editing using base editors and prime editors. Nat Plants 7: 1166–1187 [DOI] [PubMed] [Google Scholar]
- Møller IS, Gilliham M, Jha D, Mayo GM, Roy SJ, Coates JC, Haseloff J, Tester M (2009) Shoot Na+ exclusion and increased salinity tolerance engineered by cell type–specific alteration of Na+ transport in Arabidopsis. Plant Cell 21: 2163–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad Aslam M, Waseem M, Jakada BH, Okal EJ, Lei Z, Saqib HSA, Yuan W, Xu W, Zhang Q (2022) Mechanisms of abscisic acid-mediated drought stress responses in plants. Int J Mol Sci 23: 1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R, James RA (2003) Screening methods for salinity tolerance: a case study with tetraploid wheat. Plant Soil 253: 201–218 [Google Scholar]
- Munns R, James RA, Xu B, Athman A, Conn SJ, Jordans C, Byrt CS, Hare RA, Tyerman SD, Tester M, et al. (2012) Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nature Biotechnology 30, 360–364 [DOI] [PubMed] [Google Scholar]
- Nadeau E. (2007) Effects of plant species, stage of maturity and additive on the feeding value of whole-crop cereal silage. J Sci Food Agric 87: 789–801 [Google Scholar]
- Najeeb U, Bange MP, Tan DKY, Atwell BJ (2015) Consequences of waterlogging in cotton and opportunities for mitigation of yield losses. AoB Plants 7: plv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nature (2021) Revamp of UK CRISPR regulation will require public trust. Nature 591: 345. [DOI] [PubMed] [Google Scholar]
- Nelson MN, Nesi N, Barrero JM, Fletcher AL, Greaves IK, Hughes T, Laperche A, Snowdon R, Rebetzke GJ, Kirkegaard JA (2022) Strategies to improve field establishment of canola: a review. InSparks DL, ed, Advances in Agronomy, Ch. 2. Academic Press, Cambridge, MA, pp 133–177 [Google Scholar]
- Nuccio ML, Paul M, Bate NJ, Cohn J, Cutler SR (2018) Where are the drought tolerant crops? An assessment of more than two decades of plant biotechnology effort in crop improvement. Plant Sci 273: 110–119 [DOI] [PubMed] [Google Scholar]
- Ober ES, Alahmad S, Cockram J, Forestan C, Hickey LT, Kant J, Maccaferri M, Marr E, Milner M, Pinto F, et al. (2021) Wheat root systems as a breeding target for climate resilience. Theor Appl Genet 134: 1645–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata T, Ishizaki T, Fujita M, Fujita Y (2020) CRISPR/Cas9-targeted mutagenesis of OsERA1 confers enhanced responses to abscisic acid and drought stress and increased primary root growth under nonstressed conditions in rice. PLoS ONE 15: e0243376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden M, Hoefgen R, Roessner U, Persson S, Khan G (2018) Feeding the walls: how does nutrient availability regulate cell wall composition? Int J Mol Sci 19: 2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Peterson FC, Defries A, Park S-Y, Endo A, Nambara E, Volkman BF, Cutler SR (2013) Activation of dimeric ABA receptors elicits guard cell closure, ABA-regulated gene expression, and drought tolerance. Proc Natl Acad Sci USA 110: 12132–12137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palta JA, Finery IRP, Rebetzke GJ (2007) Restricted-tillering wheat does not lead to greater investment in roots and early nitrogen uptake. Field Crops Res 104: 52–59 [Google Scholar]
- Pangga IB, Hanan J, Chakraborty S (2011) Pathogen dynamics in a crop canopy and their evolution under changing climate. Plant Pathol 60: 70–81 [Google Scholar]
- Pareek A, Khurana A, Sharma AK, Kumar R (2017) An overview of signaling regulons during cold stress tolerance in plants. Curr Genomics 18: 498–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-Y, Peterson FC, Mosquna A, Yao J, Volkman BF, Cutler SR (2015) Agrochemical control of plant water use using engineered abscisic acid receptors. Nature 520: 545–548 [DOI] [PubMed] [Google Scholar]
- Parker L, Pathak T, Ostoja S (2021) Climate change reduces frost exposure for high-value California orchard crops. Sci Total Environ 762: 143971. [DOI] [PubMed] [Google Scholar]
- Parry MAJ, Andralojc PJ, Scales JC, Salvucci ME, Carmo-Silva AE, Alonso H, Whitney SM (2013) Rubisco activity and regulation as targets for crop improvement. J Exp Bot 64: 717–730 [DOI] [PubMed] [Google Scholar]
- Passioura JB (1977) Grain-yield, harvest index, and water-use of wheat. J Aust Institute Agric Sci 43, 117–120 [Google Scholar]
- Passioura JB (2020) Translational research in agriculture. Can we do it better? Crop Pasture Sci 71: 517–528 [Google Scholar]
- Patel J, Mishra A (2021) Plant aquaporins alleviate drought tolerance in plants by modulating cellular biochemistry, root-architecture, and photosynthesis. Physiol Plant 172: 1030–1044 [DOI] [PubMed] [Google Scholar]
- Phua SY, Pornsiriwong W, Chan KX, Estavillo GM, Pogson BJ (2018) Development of strategies for genetic manipulation and fine-tuning of a chloroplast retrograde signal 3'-phosphoadenosine 5'-phosphate. Plant Direct 2: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M, Raschke K (1980) Correlation between loss of turgor and accumulation of abscisic acid in detached leaves. Planta 148: 174–182 [DOI] [PubMed] [Google Scholar]
- Piltz JW, Rodham CA, Wilkins JF, Hackney BF (2021) A comparison of cereal and cereal/vetch crops for fodder conservation. Agriculture 11: 459 [Google Scholar]
- Pinto RS, Molero G, Reynolds MP (2017) Identification of heat tolerant wheat lines showing genetic variation in leaf respiration and other physiological traits. Euphytica 213: 76 [Google Scholar]
- Pirscher F, Theesfeld I (2018) The ethical dilemma with governing CRISP/Cas genome editing. InSpringer S, Grimm H, eds, Professionals in Food Chains: Ethics, Roles and Responsibilities. Wageningen Academic Publishers, Vienna, Austria, pp 419–423 [Google Scholar]
- Plett D, Safwat G, Gilliham M, Skrumsager Møller I, Roy S, Shirley N, Jacobs A, Johnson A, Tester M (2010) Improved salinity tolerance of rice through cell type-specific expression of AtHKT1;1. PLoS ONE 5: e12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JR, Gawith M (1999) Temperatures and the growth and development of wheat: a review. Eur J Agron 10: 23–36 [Google Scholar]
- Posch BC, Kariyawasam BC, Bramley H, Coast O, Richards RA, Reynolds MP, Trethowan R, Atkin OK (2019) Exploring high temperature responses of photosynthesis and respiration to improve heat tolerance in wheat. J Exp Bot 70: 5051–5069 [DOI] [PubMed] [Google Scholar]
- Posch BC, Zhai D, Coast O, Scafaro AP, Bramley H, Reich P, Ruan Y-L, Trethowan R, Way DA, Atkin O (2022) Wheat respiratory O2 consumption falls with night warming alongside greater respiratory CO2 loss and reduced biomass. J Exp Bot 73: 915–926 [DOI] [PubMed] [Google Scholar]
- Power S, Delage F, Wang G, Smith I, Kociuba G (2017) Apparent limitations in the ability of CMIP5 climate models to simulate recent multi-decadal change in surface temperature: implications for global temperature projections. Clim Dynamics 49: 53–69 [Google Scholar]
- Priest SH, Greenhalgh T (2011) Nanotechnology as an experiment in democracy: how do citizens form opinions about technology and policy? J Nanopart Res 13: 1521–1531 [Google Scholar]
- Prudent M, Dequiedt S, Sorin C, Girodet S, Nowak V, Duc G, Salon C, Maron PA (2020) The diversity of soil microbial communities matters when legumes face drought. Plant, Cell Environ 43: 1023–1035 [DOI] [PubMed] [Google Scholar]
- Prusinkiewicz P, Erasmus Y, Lane B, Harder LD, Coen E (2007) Evolution and development of inflorescence architectures. Science 316: 1452–1456 [DOI] [PubMed] [Google Scholar]
- Raman S, Mohr A (2014) A social licence for science: capturing the public or co-constructing research? Soc Epistemol 28: 258–276 [Google Scholar]
- Ran XR, Chen X, Shi L, Ashraf M, Yan FZ, Chen YQ, Xu J, Shi JX (2020) Transcriptomic insights into the roles of HSP70-16 in sepal's responses to developmental and mild heat stress signals. Environ Exp Bot 179: 104225 [Google Scholar]
- Rank AP, Koch A (2021) Lab-to-field transition of RNA spray applications - how far are we? Front Plant Sci 12: 755203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson H (1988) Effects of high temperatures on the development and yield of wheat and practices to reduce deleterious effects. InKlatt AR, ed, International Conference of Wheat Production Constraints in Tropical Environments, CIMMYT, Chang Mai, Thailand, pp 44–62 [Google Scholar]
- Rebetzke GJ, Zheng B, Chapman SC (2016) Do wheat breeders have suitable genetic variation to overcome short coleoptiles and poor establishment in the warmer soils of future climates? Funct Plant Biol 43: 961–972 [DOI] [PubMed] [Google Scholar]
- Rebetzke GJ, Botwright TL, Moore CS, Richards RA, Condon AG (2004) Genotypic variation in specific leaf area for genetic improvement of early vigour in wheat. Field Crops Res 88: 179–189 [Google Scholar]
- Regan KL, Siddique KHM, Tennant D, Abrecht DG (1997) Grain yield and water use efficiency of early maturing wheat in low rainfall Mediterranean environments. Aust J Agric Res 48: 595–603 [Google Scholar]
- Reynolds M, Foulkes J, Furbank R, Griffiths S, King J, Murchie E, Parry M, Slafer G (2012) Achieving yield gains in wheat. Plant Cell Environ 35: 1799–1823 [DOI] [PubMed] [Google Scholar]
- Reynolds M, Atkin OK, Bennett M, Cooper M, Dodd IC, Foulkes MJ, Frohberg C, Hammer G, Henderson IR, Huang B, et al. (2021) Addressing research bottlenecks to crop productivity. Trends Plant Sci 26: 607–630 [DOI] [PubMed] [Google Scholar]
- Rezaei EE, Siebert S, Ewert F (2015) Intensity of heat stress in winter wheat-phenology compensates for the adverse effect of global warming. Environ Res Lett 10: 024012 [Google Scholar]
- Richards RA, Passioura JB (1989) A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain yield in rain-fed environments. Aust J Agric Res 40: 943–950 [Google Scholar]
- Richards RA, Rebetzke GJ, Condon AG, Herwaarden AF (2002) Breeding opportunities for increasing the efficiency of water use and crop yield in temperate cereals. Crop Sci 42: 111–121 [DOI] [PubMed] [Google Scholar]
- Richards RA, Hunt JR, Kirkegaard JA, Passioura JB (2014) Yield improvement and adaptation of wheat to water-limited environments in Australia—a case study. Crop Pasture Sci 65: 676–689 [Google Scholar]
- Ritchie H, Roser M, Rosado P (2021) Crop yields. OurWorldInData.org. https://ourworldindata.org/crop-yields (June 1, 2022)
- Rodriguez-Leal D, Lemmon ZH, Man J, Bartlett ME, Lippman ZB (2017) Engineering quantitative trait variation for crop improvement by genome editing. Cell 171: 470–480 [DOI] [PubMed] [Google Scholar]
- Rooney D, Leach J, Ashworth P (2014) Doing the social in social license. Soc Epistemol 28: 209–218 [Google Scholar]
- Rosenzweig C, Elliott J, Deryng D, Ruane AC, Muller C, Arneth A, Boote KJ, Folberth C, Glotter M, Khabarov N, et al. (2014) Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc Natl Acad Sci USA 111: 3268–3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosielle AA, Hamblin J (1981) Theoretical aspects of selection for yield in stress and non-stress environments. Crop Sci 21: 943–946 [Google Scholar]
- Ryan PR, Liao MT, Delhaize E, Rebetzke GJ, Weligama C, Spielmeyer W, James RA (2015) Early vigour improves phosphate uptake in wheat. J Exp Bot 66: 7089–7100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammons RD, Ivashuta SI, Liu H, Wang D, Feng PCC, Kouranov AY, Andersen SE (2011) Polynucleotide molecules for gene regulation in plants. Monsanto Technology LLC. US 20110296556 A1Oxford University Press [Google Scholar]
- Sanchez B, Rasmussen A, Porter JR (2014) Temperatures and the growth and development of maize and rice: a review. Global Change Biol 20: 408–417 [DOI] [PubMed] [Google Scholar]
- Scafaro AP,, Atwell BJ,, Muylaert S,, Reusel BV,, Ruiz GA,, Rie JV,, Gallé A (2018) A thermotolerant variant of rubisco activase from a wild relative improves growth and seed yield in rice under heat stress. Front Plant Sci 9: 1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon TT, Doncon G (2020) Rain, rain, gone away: decreased growing-season rainfall for the dryland cropping region of the south-west of Western Australia. Crop Pasture Sci 71: 128–133 [Google Scholar]
- Sharp RE (2004) Root growth maintenance during water deficits: physiology to functional genomics. J Exp Bot 55: 2343–2351 [DOI] [PubMed] [Google Scholar]
- Shi J, Habben JE, Archibald RL, Drummond BJ, Chamberlin MA, Williams RW, Lafitte HR, Weers BP (2015) Overexpression of ARGOS genes modifies plant sensitivity to ethylene, leading to improved drought tolerance in both Arabidopsis and maize. Plant Physiol 169: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi JR, Gao HR, Wang HY, Lafitte HR, Archibald RL, Yang MZ, Hakimi SM, Mo H, Habben JE (2017) ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol J 15: 207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivaraj SM, Sharma Y, Chaudhary J, Rajora N, Sharma S, Thakral V, Ram H, Sonah H, Singla-Pareek SL, Sharma TR, et al. (2021) Dynamic role of aquaporin transport system under drought stress in plants. Environ. Exp. Bot. 184, 104367 [Google Scholar]
- Simkin AJ, Lopez-Calcagno PE, Raines CA (2019) Feeding the world: improving photosynthetic efficiency for sustainable crop production. J Exp Bot 70: 1119–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons CR, , Lafitte HR,ReimannKS, , Brugière N,Roesler K,AlbertsenMC, , GreeneTW, , Habben JE (2021) Successes and insights of an industry biotech program to enhance maize agronomic traits. Plant Sci 307: 110899. [DOI] [PubMed] [Google Scholar]
- Sinclair TR, Devi J, Shekoofa A, Choudhary S, Sadok W, Vadez V, Riar M, Rufty T (2017) Limited-transpiration response to high vapor pressure deficit in crop species. Plant Sci 260: 109–118 [DOI] [PubMed] [Google Scholar]
- Singer JW, Malone RW, Jaynes DB, Ma L (2011) Cover crop effects on nitrogen load in tile drainage from Walnut Creek Iowa using root zone water quality (RZWQ) model. Agric Water Manag 98: 1622–1628 [Google Scholar]
- Snowdon R, Wittkop B, Chen TW, Stahl A (2021) Crop adaptation to climate change as a consequence of long-term breeding. Theor Appl Genet 134: 1613–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen CJ, Morris JM, Short AH, Niyogi KK, Fleming GR (2020) Complex roles of PsbS and Xanthophylls in the regulation of nonphotochemical quenching in Arabidopsis thaliana under fluctuating light. J Phys Chem B 124: 10311–10325 [DOI] [PubMed] [Google Scholar]
- Sturgis P,, Allum N (2004) Science in society: Re-evaluating the deficit model of public attitudes. Public Underst Sci 13: 55–74 [Google Scholar]
- Sureshkumar S, Dent C, Seleznev A, Tasset C, Balasubramanian S (2016) Nonsense-mediated mRNA decay modulates FLM-dependent thermosensory flowering response in Arabidopsis. Nat Plants 2: 16055. [DOI] [PubMed] [Google Scholar]
- Svitashev S, Schwartz C, Lenderts B, Young JK, Cigan AM (2016) Genome editing in maize directed by CRISPR-Cas9 ribonucleoprotein complexes. Nat Commun 7: 13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi F, Kuromori T, Urano K, Yamaguchi-Shinozaki K, Shinozaki K (2020) Drought stress responses and resistance in plants: from cellular responses to long-distance intercellular communication. Front Plant Sci 11: 556972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukder B, Ganguli N, Matthew R, Vanloon GW, Hipel KW, Orbinski J (2021) Climate change‐triggered land degradation and planetary health: a review. Land Degrad Dev 32: 4509–4522 [Google Scholar]
- Teixeira EI, Fischer G, van Velthuizen H, Walter C, Ewert F (2013) Global hot-spots of heat stress on agricultural crops due to climate change. Agric Forest Meteorol 170: 206–215 [Google Scholar]
- Torti S, Schlesier R, Thümmler A, Bartels D, Römer P, Koch B, Werner S, Panwar V, Kanyuka K, Wirén NV, et al. (2021) Transient reprogramming of crop plants for agronomic performance. Nat Plants 7: 159–171 [DOI] [PubMed] [Google Scholar]
- Tran MT, Doan DTH, Kim J, Song YJ, Sung YW, Das S, Kim EJ, Son GH, Kim SH, Van Vu T, et al. (2021) CRISPR/Cas9-based precise excision of SlHyPRP1 domain(s) to obtain salt stress-tolerant tomato. Plant Cell Rep 40: 999–1011 [DOI] [PubMed] [Google Scholar]
- Trivedi DK, Gill SS, Tuteja N (2016) Abscisic acid (ABA): biosynthesis, regulation, and role in abiotic stress tolerance. Abiotic Stress Response in Plants 15: 311–322 [Google Scholar]
- Tuberosa R (2012) Phenotyping for drought tolerance of crops in the genomics era. Front Physiol 3: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyerman SD, McGaughey SA, Qiu J, Yool AJ, Byrt CS (2021) Adaptable and multifunctional ion-conducting aquaporins. Ann Rev Plant Biol 72: 703–736 [DOI] [PubMed] [Google Scholar]
- Uddling J, Broberg MC, Feng ZZ, Pleijel F (2018) Crop quality under rising atmospheric CO2. Curr Opin Plant Biol 45: 262–267 [DOI] [PubMed] [Google Scholar]
- Vaidya AS, Peterson FC, Yarmolinsky D, Merilo E, Verstraeten I, Park S-Y, Elzinga D, Kaundal A, Helander J, Lozano-Juste J, et al. (2017) A rationally designed agonist defines subfamily IIIA abscisic acid receptors as critical targets for manipulating transpiration. ACS Chem Biol 12: 2842–2848 [DOI] [PubMed] [Google Scholar]
- Van Eenennaam AL,, Young AE (2018) Gene editing in livestock: promise, prospects and policy. CABI Rev 2018: 1–14 [Google Scholar]
- Venkataraman G, Shabala S, Very AA, Hariharan GN, Somasundaram S, Pulipati S, Sellamuthu G, Harikrishnan M, Kumari K, Shabala L, et al. (2021) To exclude or to accumulate? Revealing the role of the sodium HKT1;5 transporter in plant adaptive responses to varying soil salinity. Plant Physiol Biochem 169: 333–342 [DOI] [PubMed] [Google Scholar]
- Wang X, Zhao C, Müller C, Wang C, Ciais P, Janssens I, Peñuelas J, Asseng S, Li T, Elliott J, et al. (2020) Emergent constraint on crop yield response to warmer temperature from field experiments. Nat Sustain 3: 908–916 [Google Scholar]
- Wasko C, Sharma A (2015) Steeper temporal distribution of rain intensity at higher temperatures within Australian storms. Nat Geosci 8: 527–529 [Google Scholar]
- Wasko C, Nathan R, Stein L, O'Shea D (2021) Evidence of shorter more extreme rainfalls and increased flood variability under climate change. J Hydrol 603: 126994 [Google Scholar]
- Wheeler SA (2009) Exploring the influences on Australian agricultural professionals' genetic engineering beliefs: an empirical analysis. J Technol Transf 34: 422–439 [Google Scholar]
- White PJ, Brown PH (2010) Plant nutrition for sustainable development and global health. Ann Botany 105: 1073–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore AP, Whalley WR (2009) Physical effects of soil drying on roots and crop growth. J Exp Bot 60: 2845–2857 [DOI] [PubMed] [Google Scholar]
- Wilson D, Jones JG (1982) Effect of selection for dark respiration rate of mature leaves on crop yields of Lolium perenne cv. S23 Ann Botany 49, 313–320 [Google Scholar]
- Winzeler M, McCullough DE, Hunt LA (1988) Genotypic differences in dark respiration of mature leaves in winter wheat (Triticum aestivum .L). Can J Plant Sci 68: 669–675 [Google Scholar]
- Woo JW, Kim J, Kwon SI, Corvalan C, Cho SW, Kim H, Kim SG, Kim ST, Choe S, Kim JS (2015) DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat Biotechnol 33: 1162–1164 [DOI] [PubMed] [Google Scholar]
- Wu A, Hammer GL, Doherty A, Von Caemmerer S, Farquhar GD (2019) Quantifying impacts of enhancing photosynthesis on crop yield. Nat Plants 5: 380–388 [DOI] [PubMed] [Google Scholar]
- Xiong DL, Ling XX, Huang JL, Peng SB (2017) Meta-analysis and dose-response analysis of high temperature effects on rice yield and quality. Environ Exp Bot 141: 1–9 [Google Scholar]
- Xiong L, Wang R-G, Mao G, Koczan JM (2006) Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiol 142: 1065–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan WK (2021) A systematic narration of some key concepts and procedures in plant breeding. Front Plant Sci 12: 724517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Liu J, Tischer SV, Christmann A, Windisch W, Schnyder H, Grill E (2016) Leveraging abscisic acid receptors for efficient water use in Arabidopsis. Proc Natl Acad Sci USA 113: 6791–6796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Han J, Kim Y-J, Song M, Yang Z, He Y, Fu R, Luo Z, Hu J, Liang W, et al. (2017) Two rice receptor-like kinases maintain male fertility under changing temperatures. Proc Natl Acad Sci USA 114: 12327–12332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaveri E, Lobell DB (2019) The role of irrigation in changing wheat yields and heat sensitivity in India. Nat Commun 10: 4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerner MC, Rebetzke GJ, Gill GS (2016) Genotypic stability of weed competitive ability for bread wheat (Triticum aestivum) genotypes in multiple environments. Crop Pasture Sci 67: 695–702 [Google Scholar]
- Zhang H, Goh NS, Wang JW, Pinals RL, Gonzalez-Grandio E, Demirer GS, Butrus S, Fakra SC, Flores AD, Zhai R, et al. (2022) Nanoparticle cellular internalization is not required for RNA delivery to mature plant leaves. Nat Nanotechnol 17: 197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HW, Si XM, Ji X, Fan R, Liu JX, Chen KL, Wang DW, Gao CX (2018) Genome editing of upstream open reading frames enables translational control in plants. Nat Biotechnol 36: 894–898 [DOI] [PubMed] [Google Scholar]
- Zhang L, Richards RA, Condon AG, Liu DC, Rebetzke GJ (2015) Recurrent selection for wider seedling leaves increases early biomass and leaf area in wheat (Triticum aestivum L.). J Exp Bot 66: 1215–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YX, Malzahn AA, Sretenovic S, Qi YP (2019) The emerging and uncultivated potential of CRISPR technology in plant science. Nat Plants 5: 778–794 [DOI] [PubMed] [Google Scholar]
- Zhao C, Piao SL, Huang Y, Wang XH, Ciais P, Huang MT, Zeng ZZ, Peng SS (2016) Field warming experiments shed light on the wheat yield response to temperature in China. Nat Commun 7: 13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Wang E, Kirkegaard JA, Rebetzke GJ (2022) Novel wheat varieties facilitate deep sowing to beat the heat of changing climates. Nat Clim Change 12: 291–296 [Google Scholar]
- Zheng B, Chapman SC, Christopher JT, Frederiks TM, Chenu K (2015) Frost trends and their estimated impact on yield in the Australian wheatbelt. J Exp Bot 66: 3611–3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng LM, Wu WY, Chen QY, Zhang GF, Gao F, Zhou YJ (2022) Integrated transcriptomics, proteomics, and metabolomics identified biological processes and metabolic pathways involved in heat stress response in jojoba. Ind Crop Prod 183: 114946 [Google Scholar]
- Zinn KE, Tunc-Ozdemir M, Harper JF (2010) Temperature stress and plant sexual reproduction: uncovering the weakest links. J Exp Bot 61: 1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziska LH, Manalo PA (1996) Increasing night temperature can reduce seed set and potential yield of tropical rice. Aust J Plant Physiol 23: 791–794 [Google Scholar]
- Zohner CM, Mo LD, Renner SS, Svenning JC, Vitasse Y, Benito BM, Ordonez A, Baumgarten F, Bastin JF, Sebald V, et al. (2020) Late-spring frost risk between 1959 and 2017 decreased in North America but increased in Europe and Asia. Proc Natl Acad Sci USA 117: 12192–12200 [DOI] [PMC free article] [PubMed] [Google Scholar]