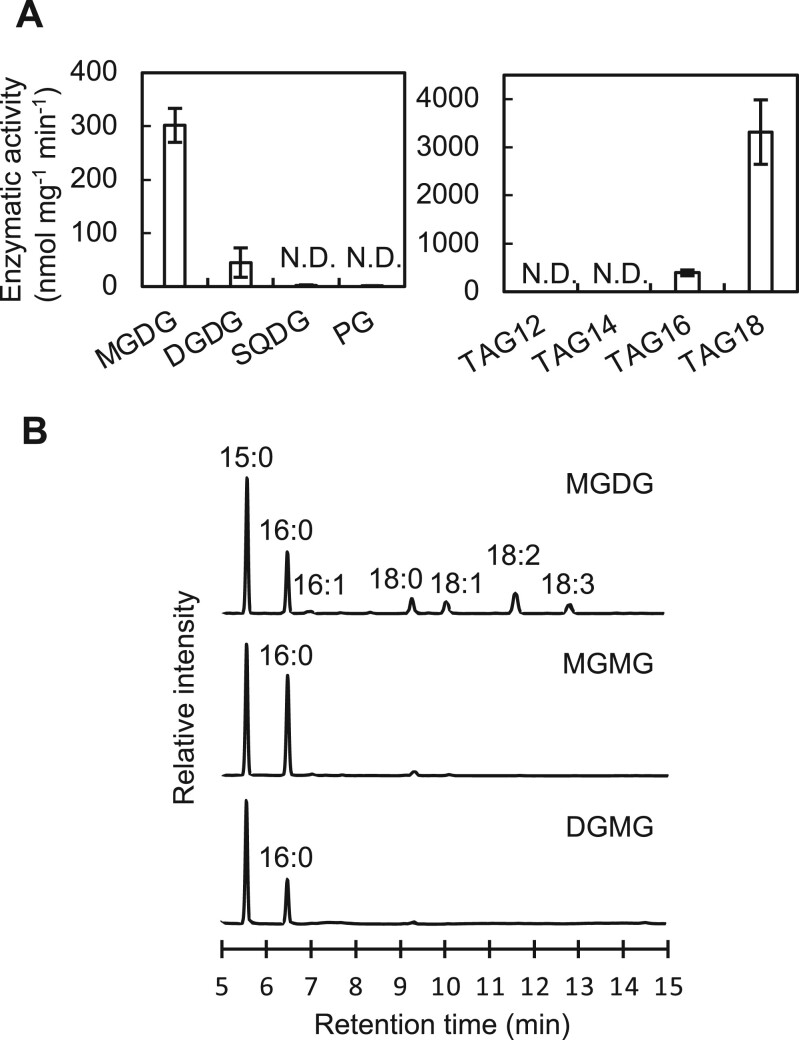
Figure 1.
Enzymatic activity of LipA in vitro and in vivo. A, Enzymatic activity of purified Sll1969 protein with a 6× His-tag (5 μg) measured with various lipid compounds (100 nmol) in 250 μL of 50 mM Tris–HCl (pH7.5) at 35°C. Reaction times were 30 min for membrane lipids (MGDG, DGDG, SQDG, and PG) and 5 min for TAG. MGDG, DGDG, SQDG, and PG used for the assay were purified from Synechocystis cells and quantified by GC. TAG compounds were purchased from Tokyo Chemical Institute (Tokyo). Values are mean ± sd of results from three independent experiments. TAG12, tri-laureateglycerol; TAG14, tri-myristateglycerol; TAG16, tri-palmitateglycerol; TAG18, tri-stearateglycerol; and N.D., not detected. B, Gas chromatograms of fatty acid methyl-esters (MEs) from MGDG purified from Synechocystis cells, and MGMG and DGMG obtained after the reaction of LipA protein with MGDG and DGDG, respectively. 15:0, pentadecanoic acid as an internal standard; 16:0, palmitic acid; 16:1, palmitoleic acid; 18:0, stearic acid; 18:1, oleic acid; 18:2, linoleic acid; and 18:3, γ-linolenic acid.