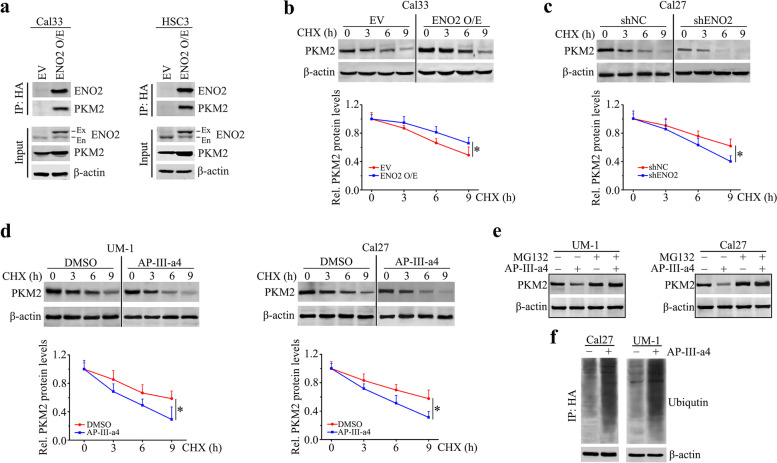
Fig. 5.
ENO2 interacts with PKM2 and protects it from ubiquitin-mediated proteasomal degradation in HNSCC cells. a The binding of ENO2 to PKM2 in ENO2 overexpressing Cal33 and HSC3 determined by IP with anti-HA antibody. b, c Effect of ENO2 overexpression (b) or knockdown (c) on the half-life of PKM2 protein determined by CHX chase assays. ENO2 overexpressing or knockdown cells were treated with 100 μg/ml CHX for the indicated hours. d Effect of AP-III-a4 on the half-life of PKM2 protein determined by CHX chase assays. Cal27 and UM-1 cells were treated with 100μg/ml CHX for the indicated hours in the presence or absence of 5µM AP-III-a4. e Effect of AP-III-a4 on PKM2 protein levels in the presence or absence of MG132. Cal27 and UM-1 cells were pretreated with 10μM MG132 for 4 h and then treated with 5µM AP-III-a4 for 24 h. f Effect of AP-III-a4 on PKM2 ubiquitination. PKM2 overexpressing Cal27 and UM-1 cells were pretreated with 10μM MG132 for 4 h before treatment of 5µM AP-III-a4. Cell lysates were IP using anti-HA antibody and immunoblotted with anti-ubiquitin antibody. β-Actin immunoblotting on total lysate is shown to normalize the input