Abstract
Aims/Introduction
Hypertriglyceridemia is common in patients with diabetes. Although the fatty acid (FA) composition of triglycerides (TGs) is suggested to be related to the pathology of diabetes and its complications, changes in the fatty acid composition caused by diabetes treatment remain unclear. This study aimed to identify short‐term changes in the fatty acid composition of plasma triglycerides after diabetes treatment.
Materials and Methods
This study was a sub‐analysis of a prospective observational study of patients with type 2 diabetes aged between 20 and 75 years who were hospitalized to improve glycemic control (n = 31). A lipidomic analysis of plasma samples on the 2nd and 16th hospital days was conducted by supercritical fluid chromatography coupled with mass spectrometry.
Results
In total, 104 types of triglycerides with different compositions were identified. Most of them tended to decrease after treatment. In particular, triglycerides with a lower carbon number and fewer double bonds showed a relatively larger reduction. The inclusion of FA 14:0 (myristic acid), as a constituent of triglyceride, was significantly associated with a more than 50%, and statistically significant, reduction (odds ratio 39.0; P < 0.001). The total amount of FA 14:0 as a constituent of triglycerides also decreased significantly, and its rate of decrease was the greatest of all the fatty acid constituents.
Conclusions
A 2 week comprehensive risk management for diabetes resulted in decreased levels of plasma triglycerides and a change in the fatty acid composition of triglycerides, characterized by a relatively large reduction in FA 14:0 as a constituent of triglycerides.
Keywords: Diabetes treatment, Lipidomics, Myristic acids
Although the fatty acid (FA) composition of triglycerides (TGs) is suggested to be related to the pathology of diabetes and its complications, changes in FAs composition caused by diabetes treatment remain unclear. This study aimed to identify short‐term changes in the FA composition of plasma TGs after comprehensive diabetes treatment. A 2‐week comprehensive diabetes treatment resulted in decreased levels of plasma TGs and a change in the FAs composition of TG, characterized by a relatively large reduction in FA 14:0 as a constituent of TGs.
INTRODUCTION
Diabetes mellitus, caused by a relative or absolute deficiency of insulin, is a group of chronic metabolic disorders characterized by hyperglycemia. Patients with diabetes also develop a variety of metabolic abnormalities, such as amino acid and lipid metabolic disorders, in addition to hyperglycemia. Hypertriglyceridemia and low HDL cholesterol levels are common in patients with diabetes. Triglycerides (TGs) comprise three fatty acid (FA) molecules with highly diverse possible FA chains. Triglycerides with different fatty acid compositions in blood are reported to have varied degrees of association with incident type 2 diabetes 1 , 2 . Plasma TG(54:2), triglyceride with 54 acyl chain carbons and two double bonds, is associated with future cardiovascular disease 3 , which is one of the major causes of mortality in patients with type 2 diabetes. These findings suggest that the fatty acid composition of plasma triglycerides is associated with the pathology of diabetes and its complications. However, the effect of diabetes treatment on the fatty acid composition remains unclear.
Intake of myristic acid, a long‐chain saturated FA (14:0), was shown to be associated with a decreased risk of incident type 2 diabetes 4 . In contrast, another study reported that serum non‐esterified FA 14:0 was positively associated with the prevalence and incidence of type 2 diabetes 5 , and a recent meta‐analysis of the association between circulating saturated fatty acids (plasma phospholipids, erythrocyte membranes fraction, or esterified or non‐esterified fatty acids in serum or whole blood) and incident type 2 diabetes revealed that circulating FA 14:0 was associated with an increased risk of incident type 2 diabetes 6 . However, the association between FA 14:0 as a constituent of triglycerides and the incidence or prevalence of type 2 diabetes remains unknown.
In most of the previous clinical lipidomic studies, triglycerides were grouped by total acyl chain carbon number and the total number of double bonds (double bond content) without identifying the constituent fatty acids. However, triglycerides with different fatty acid compositions can have the same total acyl chain carbon number and double bond content. For example, both TG(14:0/16:1/18:0) and TG(16:0/16:0/16:1) have 48 acyl chain carbons and one double bond (labeled TG(48:1)). Lee, Bamba et al. 7 reported that each triglyceride was identified successfully by supercritical fluid chromatography coupled with mass spectrometry (SFC/MS); triglycerides with different fatty acid compositions were effectively separated.
In the present study, we aimed to identify short‐term changes in the fatty acid composition of plasma triglycerides, including FA 14:0 as a constituent of triglycerides, following comprehensive risk management for diabetes using SFC/MS‐based semi‐target lipidomic techniques.
MATERIALS AND METHODS
Study design
The present study was a sub‐analysis of a prospective observational study of patients with diabetes who were hospitalized because of problems with glycemic control to investigate short‐term changes in metabolism, as described previously 8 . A lipidomic analysis of plasma samples on the 2nd and 16th hospital days with SFC/MS was conducted, and the fatty acid composition of triglycerides was compared between these 2 days. This study was approved by the Ethics Committee of Osaka University Hospital, Japan (approval number: 16374) and was conducted in accordance with the principles of the Declaration of Helsinki and current legal regulations in Japan.
Study population
Subjects were randomly recruited among patients with type 2 diabetes who were admitted to Osaka University Hospital to improve glycemic control and whose ages were between 20 and 75 years. Patients who did not receive intensive glycemic control due to severe retinopathy; patients with serum creatinine >176.80 μmoL/L (2.0 mg/dL), severe infection, or trauma; and patients in the pre‐ and postoperative periods were excluded. All participants were recruited between 2017 and 2019 and provided written informed consent after a full explanation of the study. Overall, 33 patients with diabetes were enrolled in this study 8 .
Study protocol
A medical history was obtained from all participants. Participants received comprehensive risk management for diabetes, including intensive glycemic control, as well as blood pressure, dyslipidemia, and body weight control, in the hospital, according to the Japanese treatment guideline for diabetes 9 . Blood samples were collected in the morning after fasting for about 15 h, and vital signs and weight were measured on the 2nd and 16th hospital day (hereinafter referred to as the pre‐treatment and post‐treatment periods, respectively).
For lipidomic analysis, portions of the blood samples were cooled in a freezer at 4°C immediately after collection. The samples were then centrifuged (3000 g, 10 min), and the plasma was stored at −80°C within 4 h.
Biochemical measurement
Serum triglyceride levels were measured using enzymatic assays. Estimated glomerular filtration rate was calculated according to the Statement of the Japanese Society of Nephrology 10 .
Lipidomic measurement
Plasma samples were prepared for lipid extraction using the Bligh and Dyer method 11 , with minor modifications. Lipids were extracted from plasma (30 μL) with 470 μL of methanol containing 0.70 nmol TG 15:0/18:1 (d7)/15:0 as the internal standard. The samples were then vigorously mixed for 1 min and centrifuged at 16,000 g for 5 min at 4°C. The resultant supernatant (400 μL) was collected. After mixing with chloroform (400 μL) and water (224 μL), the aqueous and organic layers were separated by mixing and centrifuging at 16,000 g at 4°C for 5 min. The organic layer (bottom, 300 μL), obtained by phase separation, was dried under a nitrogen stream, then stored at −80°C until analysis. Prior to the analysis, the dried sample was reconstituted in methanol/chloroform (1/1, v/v, 200 μL).
The analytical conditions for supercritical fluid chromatography tandem mass spectrometry (SFC/MS/MS) analysis were performed as described previously 12 , 13 . The SFC (Nexera UC system, Shimadzu, Japan) conditions were as follows: column, Acquity UPC2 HSS C18 SB column (3.0 mm i.d. × 100 mm, 1.7 μm particle size, Waters); injection volume, 2 μL; column temperature, 50°C; mobile phase A, supercritical carbon dioxide; mobile phase B (modifier) and make‐up pump solvent; methanol/water (95/5, v/v) with 0.1% (w/v) ammonium acetate; flow rate of mobile phase, 1.0 mL/min; flow rate of make‐up pump, 0.1 mL/min; and back pressure regulator, 10 MPa. The gradient conditions were as follows: 0–50% B, 0–25 min; 50% B, 25–28 min; and 0% B, 28.1–30 min. The triple quadrupole mass spectrometer (TQMS, LCMS‐8060, Shimadzu, Japan) analysis conditions were as follows: polarity, positive and negative ionization; electrospray voltage, 4 kV in the positive ion mode for triglyceride analysis and −3.5 kV in the negative ion mode for FAs analysis; nebulizer gas flow rate, 3.0 L/min; drying gas flow rate, 10.0 L/min; desolvation line temperature, 250 °C; heat block temperature, 400 °C; and detector voltage, 2.16 kV. The multiple reaction monitoring (MRM) parameters per time period were as follows: limit on the number of MRM transitions, 150; dwell time, 2 ms; and pause time, 2 ms. Data processing was performed using LabSolution software version 5.99 SP2 (Shimadzu, Kyoto, Japan).
A reference sample was prepared by mixing equal amounts (10 μL each) of plasma extracts. The 20 FA constituents (i.e., FA 14:0, FA 16:0, FA 16:1, FA 18:0, FA 18:1, FA 18:2, FA 20:0, FA 20:1, FA 20:2, FA20:3, FA 20:4, FA 20:5, FA 22:0, FA 22:1, FA 22:4, FA 22:5, FA 22:6, FA 24:0, FA 26:0, and FA 28:0) of the triglycerides in plasma were determined by SFC/MS/MS analysis of the hydrolyzed reference sample 12 . To determine the target triglyceride molecules in plasma, a reference sample was analyzed using the in‐house MRM library for 1,540 targeted triglyceride molecules 14 . Targeted quantitative analysis of 104 selected triglycerides in all the samples was performed using the selected MRM method 13 , 14 .
Validation for this SFC/MS method is shown in Table S1 and Figure S1, indicating good repeatability and accuracy.
Statistical analysis
Clinical data are expressed as mean and standard deviation if normally distributed, or as median and interquartile range if log‐normally distributed. Categorical variables are expressed as counts and percentages. Statistical significance was set at P < 0.05. Paired t‐tests were used to compare data between pre‐ and post‐treatment periods. If the variables were log‐normally distributed, tests were used after log‐transformation.
To investigate the overview of qualitative changes in triglyceride, we calculated the fold‐change value (the median of the ratios between post‐treatment value and pre‐treatment value) of each triglyceride and assessed the association of the fold‐change value with total acyl chain carbon number and double bond content (total number of double bonds).
To compare the fatty acid composition of triglycerides between the pre‐ and post‐treatment periods, triglyceride values were compared using paired t‐tests after log‐transformation. To account for multiple testing, we used the Benjamini‐Hochberg method; that is, we calculated the adjusted P‐values (q‐values) with the significance level set at 0.05. A volcano plot was constructed using the q‐values and fold‐change values. We then selected the triglycerides with significantly different changes upon paired t‐tests and whose fold‐change values were not above 50% nor below 150%.
Next, we calculated the value of each fatty acid as a constituent of triglycerides by summing up the products of each triglyceride value and the number of fatty acids that constitute the triglyceride. We then compared the values of each fatty acid as a constituent of triglyceride between the pre‐ and post‐treatment periods, similar to each triglyceride value. Moreover, the same comparison was performed among the obese and non‐obese patients.
Finally, Pearson's correlations between the differences of fatty acids as constituents of triglyceride between pre‐ and post‐treatment and those in clinical factors were analyzed, for the purpose of evaluating associations between the changes in fatty acids as constituents of triglyceride and the clinical factors. In addition to fatty acids as constituents of triglyceride, we evaluated the associations of total triglyceride, which were calculated by summing all triglycerides.
RESULTS
Effect of comprehensive risk management for diabetes on clinical parameters
Two patients withdrew consent after participation; therefore, 31 patients were included in the final analysis. The baseline clinical characteristics of the study participants have been described previously 8 . Briefly, the age of the participants was 63.9 ± 10.5 (mean ± standard deviation) years, 41.9% of the participants were men, glycated hemoglobin (HbA1c) was 9.1 ± 2.1%, and the body mass index (BMI) was 26.7 ± 5.1 kg/m2. Most of the patients were treated with insulin (77.4%) or biguanide (64.5%) after admission, and insulin users were increased (Table S2). Comprehensive risk management for diabetes resulted in a statistically significant reduction in fasting plasma glucose, HbA1c, body weight, aspartate aminotransferase, γ‐glutamyl transpeptidase, highly sensitive C‐reactive protein, total cholesterol, low‐density lipoprotein‐cholesterol, and high‐density lipoprotein‐cholesterol levels. Serum triglyceride levels, measured by the enzymatic assay, also significantly decreased from 1.41 (0.92–2.59) to 1.06 (0.79–1.45) mmol/L, as reported previously 8 (median with interquartile range).
Change in triglycerides assessed by lipidomic measurement
All 104 targeted triglycerides with different fatty acids compositions were identified using SFC/MS (Table S3). We identified an association between the fold‐change value in triglycerides and their total acyl chain carbon number (r = 0.762, P < 0.001; Figure 1a) and double bond content (r = 0.474, P < 0.001; Figure 1b). In other words, triglycerides with relatively lower carbon numbers and fewer double bonds decreased more drastically after treatment.
Figure 1.
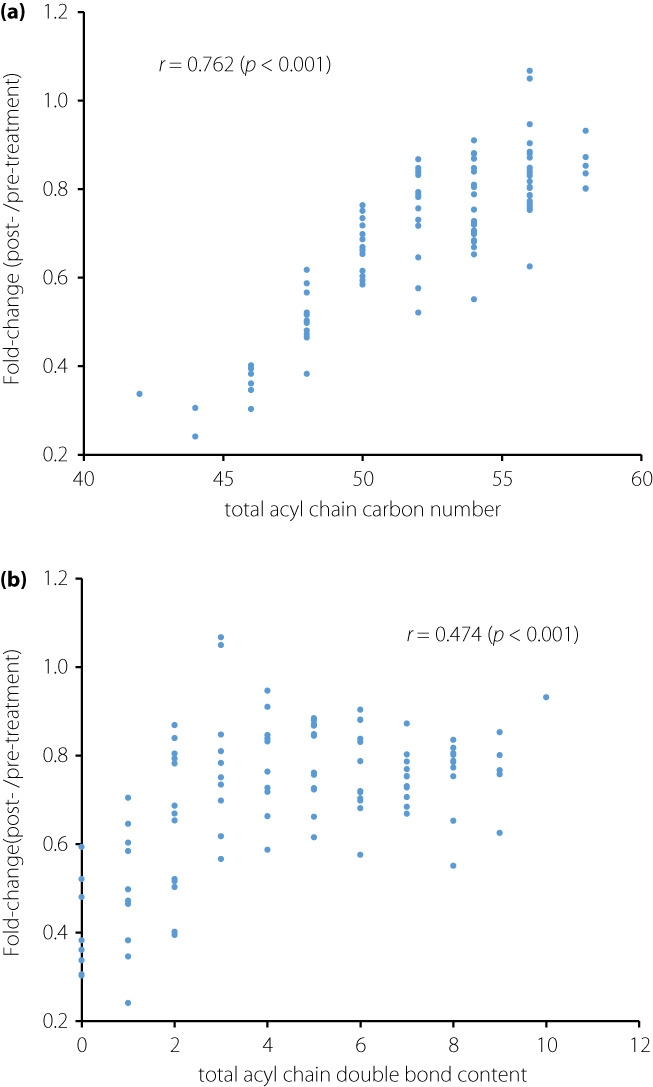
Associations between the fold‐change values (the medians of ratios between post‐treatment value/pre‐treatment value) in triglycerides (TGs), and (a) total acyl chain carbon number and (b) double bond content. The Spearman's rank correlations were used to calculate correlation coefficient r.
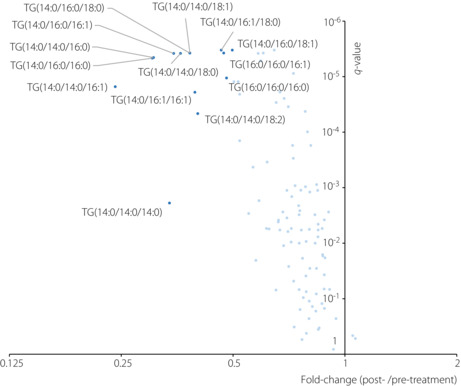
As shown in Figure 2, paired t‐tests comparing the triglyceride values before and after treatment showed that most of the identified triglycerides tended to decrease after treatment. Among them, a total of 14 triglycerides showed a statistically significant decrease and more than a 50% reduction (dark blue points in Figure 2). Among them, 12 triglycerides (85.7% of them) had FA 14:0 as a constituent, and seven triglycerides (50% of them) had FA 16:0. On the other hand, among the other 90 triglycerides (light blue points in Figure 2), which did not show drastic reduction after treatment, only 12 triglycerides (13.3%) had FA 14:0. Notably, the inclusion of FA 14:0 as a constituent was significantly associated with statistically significant and more than 50% reduction in triglycerides (odds ratio 39.0; P < 0.001). In contrast, the inclusion of FA 16:0 was not (odds ratio 1.31; P = 0.640).
Figure 2.
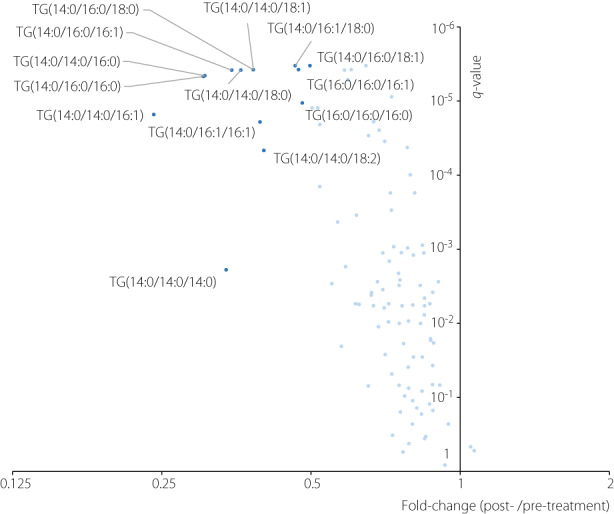
Volcano plot showing the results of the paired t‐tests comparing triglycerides (TG) values of the pre‐ and post‐treatment. The x‐axis represents the fold‐change values (the medians of ratios between post‐treatment value/pre‐treatment value), whereas the y‐axis represents the q‐values (P‐values adjusted using the Benjamini–Hochberg method). Dark blue points represent the TGs with a significant difference (q‐value ≦ 0.05) and 50% change (fold‐change≧1.5 or ≦0.5). Light blue points represent the other TGs.
As shown in Figure 3, comparing the values of each fatty acid as a constituent of triglycerides between the pre‐ and post‐treatment periods showed that FA 14:0 significantly decreased, and its decrease rate was the largest among all the fatty acid constituents. Other fatty acids in the de novo lipogenesis (DNL) pathway, such as FA 16:0, FA 18:0, and FA 16:1, also showed relatively large decrease rates. These DNL‐related fatty acids showed a statistically significant decrease among the obese patients, while their decrease was not significant among the non‐obese patients (Figure 4).
Figure 3.
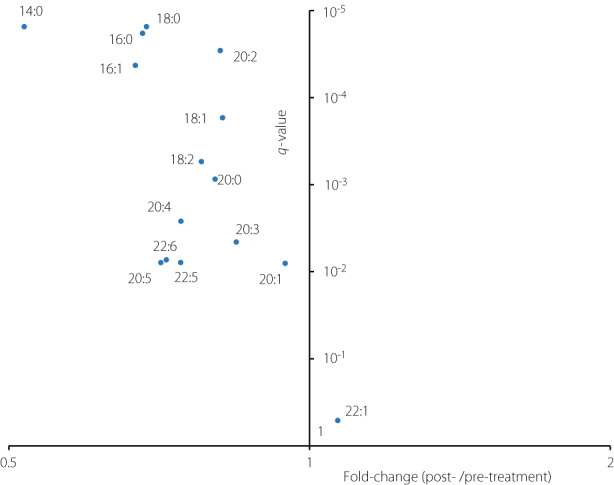
Volcano plot showing the results of the paired t‐tests comparing the values of each fatty acid as a constituent of triglycerides (TG) between the pre‐ and post‐treatment period. The x‐axis represents the fold‐change values (the medians of ratios between post‐treatment value/pre‐treatment value), whereas the y‐axis represents the q‐values (adjusted P‐values using the Benjamini–Hochberg method).
Figure 4.
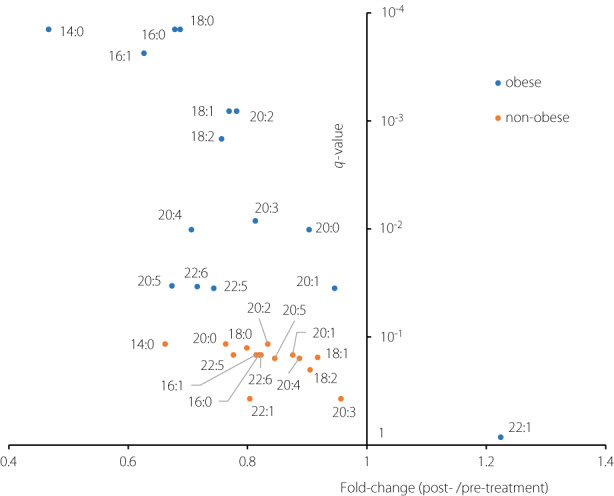
Comparison of the results of the paired t‐tests comparing the values of each fatty acid as a constituent of triglyceride (TG) between the pre‐ and post‐treatment period among the obese patients (body mass index [BMI] ≥ 25; n = 21) with those among the non‐obese patients (BMI < 25; n = 10). The x‐axis represents the fold‐change values (the medians of ratios of post‐treatment value/pre‐treatment value), whereas the y‐axis represents the q‐values (adjusted P‐values using the Benjamini–Hochberg method).
Associations between delta changes in fatty acids as constituents of triglycerides before and after treatment and those in clinical parameters are shown in Table 1. Changes in FA 16:0, FA 16:1, FA 18:0, FA 18:1, and FA 18:2 as constituents of triglycerides had a moderate association with those in HbA1c levels, while those in FA 14:0 had no statistically significant association.
Table 1.
Associations between the changes in fatty acids as constituents of triglycerides and clinical factors
| ΔBMI | ΔAST | ΔALT | Δγ‐GTP | ΔFPG | ΔHbA1c | ΔADN | |
|---|---|---|---|---|---|---|---|
| ΔFA 14:0 | 0.197 | 0.287 | 0.174 | 0.222 | 0.119 | 0.122 | 0.048 |
| ΔFA 16:0 | 0.138 | 0.207 | 0.029 | 0.252 | 0.266 | 0.486* | −0.233 |
| ΔFA 16:1 | 0.114 | 0.267 | 0.106 | 0.204 | 0.206 | 0.358* | −0.071 |
| ΔFA 18:0 | 0.126 | 0.250 | 0.021 | 0.225 | 0.241 | 0.489* | −0.215 |
| ΔFA 18:1 | 0.028 | 0.165 | 0.020 | 0.255 | 0.290 | 0.494* | −0.218 |
| ΔFA 18:2 | 0.035 | 0.056 | −0.001 | 0.306 | 0.325 | 0.496* | −0.266 |
| Δtotal TG | 0.093 | 0.180 | 0.032 | 0.266 | 0.276 | 0.471* | −0.213 |
Pearson's correlation coefficients between the differences in FAs as constituents of TGs before and after treatment and those of clinical factors are presented. The major FAs that constitute triglycerides and total triglyceride are shown in the first column, and major clinical factors are shown in the first row. *P < 0.05. ADN, adiponectin; ALT, alanine transaminase; AST, aspartate aminotransferase; BMI, body mass index; FA, fatty acid; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; TG, triglyceride; γ‐GTP, γ‐glutamyl transpeptidase.
DISCUSSION
In the present study, triglycerides with relatively lower carbon numbers and fewer double bonds (especially triglycerides with 42–48 total carbon numbers or 0–2 total double bonds) showed a larger reduction after comprehensive risk management for diabetes. This finding is consistent with that of previous studies. Schwab et al. 15 examined alterations in the fatty acid composition of plasma triglyceride induced by body weight reduction after diet therapy in nine individuals and showed that plasma triglycerides with fewer carbon numbers and those with lower double bond content had a relatively larger decrease after a 33 week intervention. Further, a decrease in plasma triglycerides containing relatively short‐chain saturated fatty acids was correlated with increased insulin sensitivity 15 . Similarly, triglycerides with lower carbon number and lower double bond content strongly correlated with insulin resistance and an increased risk of diabetes, compared with triglyceride with a higher carbon number and a higher double bond content 16 , 17 .
However, in these previous clinical lipidomic studies, triglycerides were grouped by the total acyl chain carbon number and the total number of double bonds (double bond content) without assessing fatty acid constituents. One of the strengths of the present study is that we identified triglycerides with different fatty acid compositions separately using SFC/MS‐based semi‐target lipidomic techniques 4 and assessed the change in each triglyceride separately.
The present study showed a relatively large reduction of FA 14:0, FA 16:0, FA 16:1, and FA 18:0 as constituents of triglycerides. Since these fatty acids belong to the DNL pathway, the larger reduction of triglycerides with relatively lower carbon numbers and fewer double bonds might be related to DNL. Notably, the decrease in these DNL‐related fatty acids was statistically significant among the obese patients only. The lack of significance in the non‐obese patients could be due to their small sample size, but obese patients tended to have a larger decrease rate of these triglycerides compared with the non‐obese patients. In obese individuals, hepatic DNL is reported to contribute substantially to intrahepatic triglyceride than in lean individuals 18 . Weight reduction can improve peripheral insulin resistance, thus reducing circulating insulin. In effect, reduced circulating insulin decreases hepatic DNL, which might have contributed to the larger reduction of triglycerides with relatively lower carbon numbers and fewer double bonds, especially in the obese patients. However, the influence of anti‐diabetic medications on the circulating insulin in the present study is unclear since the change of circulating insulin depends on the class of medications. Given the small sample size, we could not assess the associations between the class of medications and the change in the fatty acid composition of plasma triglycerides.
The present study also revealed that triglycerides containing FA 14:0 (myristic acid) as a constituent were drastically reduced after comprehensive risk management for diabetes. This finding is consistent with those of previous studies. For example, Schwab et al. 15 also reported that plasma levels of esterified FA 14:0 showed a relatively larger reduction after body weight reduction compared with that of other esterified fatty acids. Similarly, Guerendiain et al. 19 reported that body weight reduction induced a decrease in FA 14:0 in overweight adolescents and that subjects with greater weight reduction displayed a significant decrease in FA 14:0. They also revealed that a decrease in FA 14:0 is associated with a reduction in insulin levels 19 . While the decrease in most of the major fatty acid constituents of triglycerides was associated with improvement of glycemic control in the present study, that in FA 14:0 was not. Although the association between changes in FA 14:0 and that in the BMI was not statistically significant in the present study, this might be due to our small sample size. Considering the results of these previous studies, the decrease in FA 14:0 as a constituent of triglycerides may have been associated with body weight change. One possible mechanism is the involvement of adiponectin. A previous cross‐sectional study revealed a negative association between serum adiponectin and FA 14:0 in serum phospholipids 20 . However, other clinical studies reported no associations between serum adiponectin and FA 14:0 derived from erythrocyte membrane 21 , plasma esterified FA 22 , 23 , and adipose tissue 23 . In the present study, no association was observed between the change of serum adiponectin and that of FA 14:0 as a constituent of triglycerides. Therefore, the association between weight reduction and a decrease in FA 14:0 as a constituent of triglycerides might not be adiponectin‐mediated.
Because blood samples were collected under fasting conditions, plasma triglycerides measured in this study were mainly derived from very low‐density lipoprotein particles, and thus were considered to reflect liver fat content. Therefore, it is possible that adipose tissue lipolysis, DNL in the liver, and the effect of diet changed the fatty acid composition of plasma triglycerides, although the mechanism underlying the relatively large decrease in FA 14:0 as a constituent of triglycerides remains unclear.
Lipolysis of adipose tissue is a major source of fatty acids stored in the liver. Insulin suppresses adipose tissue lipolysis. A previous study investigating the changes in individual free fatty acids during an oral glucose tolerance test indicated a remarkable decrease in saturated and monounsaturated fatty acids and a relative increase in polyunsaturated fatty acids 24 . Accordingly, the degree of suppression of adipose tissue lipolysis by insulin may differ among fatty acid species. The increased insulin action in adipose tissue due to anti‐diabetic medications or weight reduction can suppress adipose tissue lipolysis, and the difference in the degrees of suppression among fatty acids may have caused the change in the fatty acid composition of triglycerides.
Fatty acid synthesis in the liver can also affect the fatty acid composition of triglycerides. Key enzymes involved in the biosynthesis of fatty acids with relatively lower carbon number (14–18) and those with fewer double bonds (0–1) are fatty acid synthase (FAS), elongation of long‐chain fatty acid family member 6 (Elovl6), and stearoyl‐CoA desaturase 1 (SCD1), all of which are regulated by sterol regulatory element‐binding protein‐1c (SREBP‐1c) 25 , 26 , 27 . Acetyl‐CoA is mainly converted to FA 16:0 (palmitic acid) by FAS, followed by elongation and desaturation. The elongation and desaturation enzymes are Elovl6 and SCD1, respectively. Fatty acid synthase also produces minor amounts of FA 14:0 28 , which are converted to FA 16:0 by Elovl6. As mentioned earlier, weight reduction seems to have decreased the circulating insulin, and anti‐diabetic medications might also have changed it in the present study. Since insulin stimulates these FA‐synthesis‐related enzymes via SREBP‐1c 25 , the subsequent change of circulating insulin can affect the fatty acid composition of triglycerides.
Dietary intake affects the fatty acid composition of blood triglycerides and other lipid classes 29 . Although high‐fat dairy products contain relatively high amounts of myristic acid, dietary fat from other sources contains only low amounts. In the present study, the influence of dietary fat intake on the change in FA 14:0 in triglyceride was unclear, since we have no information on the intake of high‐fat dairy products.
FA 14:0 has both negative and positive effects on mammals. FA 14:0 can predict NASH 30 and potentiate FA 16:0‐induced lipotoxicity in hepatocytes 31 . In contrast, it was also shown to enhance diacylglycerol kinase δ‐dependent glucose uptake in a myotube model 32 . Furthermore, chronic administration of FA 14:0 improved hyperglycemia and insulin resistance in skeletal muscles in an animal model of type 2 diabetes 33 . The fatty acid composition of plasma triglycerides reflects liver fat content, as well as fatty acid transport to peripheral tissue. A relatively large decrease in FA 14:0 as a constituent of triglycerides following comprehensive risk management for diabetes could lead to attenuation of the noxious effect of FA 14:0 on the liver, as well as its beneficial effect on skeletal muscle. However, the beneficial insulin resistance amelioration effect of FA 14:0 on skeletal muscle may not necessarily be required once glycemic control improves. Therefore, a large decrease in FA 14:0 as a constituent of triglycerides after comprehensive risk management for diabetes mainly reflects its beneficial effect on the whole body.
The present study has several limitations. First, there is no detailed patient information about lifestyle‐related factors, such as diet, physical activity, and alcohol consumption. These factors have a significant influence on plasma lipid levels. We also did not have detailed information about the fatty acid composition of the diet given to patients during their hospital stay, although the amount and composition of major nutrients in the food portions given to patients at the hospital were determined by the attending physician according to the patients' respective physical data and diabetes complications. Second, the present study was a pilot study to discover potentially promising hypotheses; the sample size was too small to draw a definite conclusion. Therefore, further studies with larger sample sizes are warranted. Third, we could not assess the change of circulating insulin level, as the majority of the study participants were treated with insulin.
In conclusion, most plasma triglycerides decreased after a 2 week comprehensive risk management for diabetes. Furthermore, FA 14:0 as a constituent of triglycerides showed the largest reduction among the detected fatty acid constituents.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: The research protocol was approved by the Ethics Committee of Osaka University Hospital.
Informed consent: Written informed consent was obtained from all the participants after a full explanation of the study.
Registry and the registration no. of the study/trial: 1 May 2017, No. 16374.
Animal studies: N/A.
Supporting information
Figure S1 | Intra‐ and inter‐day variation in the concentrations of 104 triglycerides (TGs) detected in human plasma
Table S1 | Spike‐and‐recovery test for determination of triglycerides (TGs)
Table S2 | Anti‐diabetic medications before and during the comprehensive risk management for diabetes
Table S3 | Changes in all 104 identified triglycerides (TGs) after comprehensive risk management for diabetes
ACKNOWLEDGMENT
The authors thank study participants for their participation. The authors also wish to thank Editage (www.editage.com) for English language editing.
This study was supported by the AMED‐CREST from the Japan Agency for Medical Research and Development (AMED) (grant numbers: JP19gm0710005, JP21gm0910013, and JP21gm1010010), the Manpei Suzuki Diabetes Foundation, and the Japan Diabetes Foundation.
References
- 1. Razquin C, Toledo E, Clish CB, et al. Plasma lipidomic profiling and risk of Type 2 diabetes in the PREDIMED trial. Diabetes Care 2018; 41: 2617–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu J, Lam SM, Wan Q, et al. High‐coverage targeted lipidomics reveals novel serum lipid predictors and lipid pathway dysregulation antecedent to Type 2 diabetes onset in normoglycemic Chinese adults. Diabetes Care 2019; 42: 2117–2126. [DOI] [PubMed] [Google Scholar]
- 3. Stegemann C, Pechlaner R, Willeit P, et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population‐based Bruneck study. Circulation 2014; 129: 1821–1831. [DOI] [PubMed] [Google Scholar]
- 4. Ericson U, Hellstrand S, Brunkwall L, et al. Food sources of fat may clarify the inconsistent role of dietary fat intake for incidence of type 2 diabetes. Am J Clin Nutr 2015; 101: 1065–1080. [DOI] [PubMed] [Google Scholar]
- 5. Lu Y, Wang Y, Zou L, et al. Serum lipids in association with type 2 diabetes risk and prevalence in a Chinese population. J Clin Endocrinol Metab 2018; 103: 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang L, Lin JS, Aris IM, et al. Circulating saturated fatty acids and incident type 2 diabetes: a systematic review and meta‐analysis. Nutrients 2019; 11: 998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee JW, Uchikata T, Matsubara A, et al. Application of supercritical fluid chromatography/mass spectrometry to lipid profiling of soybean. J Biosci Bioeng 2012; 113: 262–268. [DOI] [PubMed] [Google Scholar]
- 8. Taya N, Katakami N, Omori K, et al. Evaluation of change in metabolome caused by comprehensive diabetes treatment: a prospective observational study of diabetes inpatients with gas chromatography/mass spectrometry‐based non‐target metabolomic analysis. J Diabetes Investig 2021; 12: 2232–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. The Japan Diabetes Society . The Japanese Treatment Guideline for Diabetes 2016–2017. Tonyobyo Chiryo guide 2016–2017. Bunkodo: The Japan Diabetes Society, 2016. (Japanese). [Google Scholar]
- 10. Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 11. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959; 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 12. Ogawa T, Izumi Y, Kusumoto K, et al. Wide target analysis of acylglycerols in miso (Japanese fermented soybean paste) by supercritical fluid chromatography coupled with triple quadrupole mass spectrometry and the analysis of the correlation between taste and both acylglycerols and free fatty acids. Rapid Commun Mass Spectrom 2017; 31: 928–936. [DOI] [PubMed] [Google Scholar]
- 13. Fushimi T, Izumi Y, Takahashi M, et al. Dynamic metabolome analysis reveals the metabolic fate of medium‐chain fatty acids in AML12 cells. J Agric Food Chem 2020; 68: 11997–12010. [DOI] [PubMed] [Google Scholar]
- 14. Takeda H, Izumi Y, Takahashi M, et al. Widely‐targeted quantitative lipidomics method by supercritical fluid chromatography triple quadrupole mass spectrometry. J Lipid Res 2018; 59: 1283–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwab U, Seppänen‐Laakso T, Yetukuri L, et al. Triacylglycerol fatty acid composition in diet‐induced weight loss in subjects with abnormal glucose metabolism ‐ The GENOBIN study. PLoS One 2008; 3: e2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rhee EP, Cheng S, Larson MG, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 2011; 121: 1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kopprasch S, Dheban S, Schuhmann K, et al. Detection of independent associations of plasma lipidomic parameters with insulin sensitivity indices using data mining methodology. PLoS One 2016; 11: e0164173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith GI, Shankaran M, Yoshino M, et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J Clin Invest 2020; 130: 1453–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guerendiain M, Montes R, López‐Belmonte G, et al. Changes in plasma fatty acid composition are associated with improvements in obesity and related metabolic disorders: a therapeutic approach to overweight adolescents. Clin Nutr 2018; 37: 149–156. [DOI] [PubMed] [Google Scholar]
- 20. Kurotani K, Sato M, Yasuda K, et al. Even‐ and odd‐chain saturated fatty acids in serum phospholipids are differentially associated with adipokines. PLoS One 2017; 12: e0178192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Santos S, Oliveira A, Pinho C, et al. Fatty acids derived from a food frequency questionnaire and measured in the erythrocyte membrane in relation to adiponectin and leptin concentrations. Eur J Clin Nutr 2014; 68: 555–560. [DOI] [PubMed] [Google Scholar]
- 22. Fernández‐Real JM, Vendrell J, Ricart W. Circulating adiponectin and plasma fatty acid profile. Clin Chem 2005; 51: 603–609. [DOI] [PubMed] [Google Scholar]
- 23. Hernandez‐Morante JJ, Milagro FI, Larque E, et al. Relationship among adiponectin, adiponectin gene expression and fatty acids composition in morbidly obese patients. Obes Surg 2007; 17: 516–524. [DOI] [PubMed] [Google Scholar]
- 24. Zhao X, Peter A, Fritsche J, et al. Changes of the plasma metabolome during an oral glucose tolerance test: is there more than glucose to look at? Am J Physiol Endocrinol Metab 2009; 296: E384–E393. [DOI] [PubMed] [Google Scholar]
- 25. Shimomura I, Bashmakov Y, Ikemoto S, et al. Insulin selectively increases SREBP‐1c mRNA in the livers of rats with streptozotocin‐induced diabetes. Proc Natl Acad Sci USA 1999; 96: 13656–13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moon YA, Shah NA, Mohapatra S, et al. Identification of a mammalian long chain fatty acyl elongase regulated by sterol regulatory element‐binding proteins. J Biol Chem 2001; 276: 45358–45366. [DOI] [PubMed] [Google Scholar]
- 27. Tabor DE, Kim JB, Spiegelman BM, et al. Identification of conserved cis‐elements and transcription factors required for sterol‐regulated transcription of stearoyl‐CoA desaturase 1 and 2. J Biol Chem 1999; 274: 20603–20610. [DOI] [PubMed] [Google Scholar]
- 28. Rioux V, Catheline D, Legrand P. In rat hepatocytes, myristic acid occurs through lipogenesis, palmitic acid shortening and lauric acid elongation. Animal 2007; 1: 820–826. [DOI] [PubMed] [Google Scholar]
- 29. Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008; 47: 348–380. [DOI] [PubMed] [Google Scholar]
- 30. Tomita K, Teratani T, Yokoyama H, et al. Plasma free myristic acid proportion is a predictor of nonalcoholic steatohepatitis. Dig Dis Sci 2011; 56: 3045–3052. [DOI] [PubMed] [Google Scholar]
- 31. Martínez L, Torres S, Baulies A, et al. Myristic acid potentiates palmitic acid‐induced lipotoxicity and steatohepatitis associated with lipodystrophy by sustaning de novo ceramide synthesis. Oncotarget 2015; 6: 41479–41496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wada Y, Sakiyama S, Sakai H, et al. Myristic acid enhances diacylglycerol kinase δ‐dependent glucose uptake in myotubes. Lipids 2016; 51: 897–903. [DOI] [PubMed] [Google Scholar]
- 33. Takato T, Iwata K, Murakami C, et al. Chronic administration of myristic acid improves hyperglycaemia in the Nagoya–Shibata–Yasuda mouse model of congenital type 2 diabetes. Diabetologia 2017; 60: 2076–2083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Intra‐ and inter‐day variation in the concentrations of 104 triglycerides (TGs) detected in human plasma
Table S1 | Spike‐and‐recovery test for determination of triglycerides (TGs)
Table S2 | Anti‐diabetic medications before and during the comprehensive risk management for diabetes
Table S3 | Changes in all 104 identified triglycerides (TGs) after comprehensive risk management for diabetes


