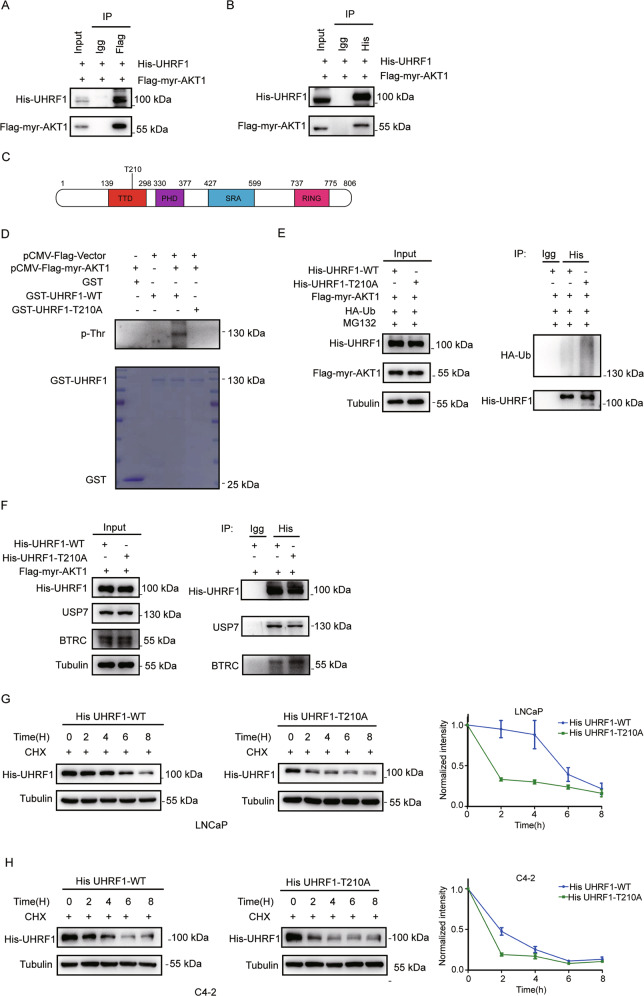
Fig. 4. AKT induces UHRF1 phosphorylation via the threonine 210 residue, and sustains UHRF1 protein stability via binding deubiquitinase USP7.
A, B HEK-293T cells were co-transfected with the plasmids encoding His-UHRF1 and Flag-myr-AKT1. AKT, or UHRF1 protein was immunoprecipitated by using Anti-Flag antibody (A) or Ant-His antibody (B), The AKT or UHRF1 protein was assessed by western blotting. C An AKT consensus motif (RXRXXS/T) was identified in the TTD domain of UHRF1 protein. D The wild-type or mutant of UHRF1 (GST-UHRF1-WT or T210A) or FLAG-myr-AKT1 was constructed, and the recombinant proteins were purified. A in vitro kinase assay was performed by incubating the purified proteins with the magnetic beads, and the phosphorylated UHRF1 was detected by immunoblotting with anti-threonine phosphorylation antibody. E HEK-293T cells were co-transfected with the plasmids encoding His-UHRF1(WT or T210A), Flag-myr-AKT1 and HA-ubiquitin for 48 h, and then treated with 50 μM MG132 for 8 h. UHRF1 protein was immunoprecipitated by using anti-His antibody, and the ubiquitinated-UHRF1 were detected with anti-HA antibody by western blotting. F HEK-293T cells were co-transfected with the plasmids encoding His-UHRF1(WT or T210A), Flag-myr-AKT1 for 48 h. UHRF1 protein was immunoprecipitated with anti-His antibody, and UHRF1 or BTRC protein was detected by western blotting. G, H LNCaP and C4-2 cells were transfected with the plasmids encoding His-UHRF1-WT or T210A for 48 h, and then treated with 50 uM cycloheximide (CHX) for the indicated time. The protein level of UHRF1 were detected with anti-His antibody by western blotting. The presented results were representative of experiments repeated at least three times. Data was represented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.