TABLE 2.
Green oxidation of primary alcohol derivatives to aldehyde derivatives using CCPSF NPs under MW irradiation.
| Primary alcohol (A) | Aldehyde (B) | Time (min) | Yield (%) | Found M. P. ( ) | Reported M. P. ( ) (Ghalehbandi et al., 2020) | |
|---|---|---|---|---|---|---|
| 1 |

|

|
2 | 91 | Liq. | Liq. |
| 2 |

|

|
3 | 92 | Liq. | Liq. |
| 3 |
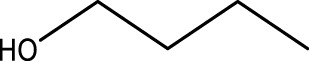
|

|
2 | 90 | Liq. | Liq. |
| 4 |

|

|
2 | 91 | Liq. | Liq. |
| 5 |

|

|
2 | 91 | Liq. | Liq. |
| 6 |

|

|
2 | 95 | Liq. | Liq. |
| 7 |

|

|
2 | 100 | Liq. | Liq. |
| 8 |
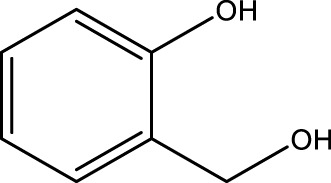
|

|
2 | 100 | Liq. | Liq. |
| 9 |

|

|
2 | 93 | Liq. | Liq. |
| 10 |

|
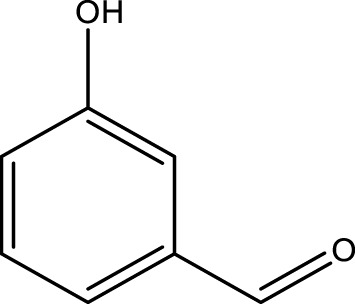
|
2 | 98 | 101–103 | 104 |
| 11 |

|
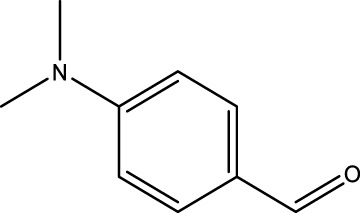
|
2 | 97 | 76–77 | 77 |
| 12 |

|

|
2 | 100 | Liq. | Liq. |
| 13 |

|

|
2 | 100 | Liq. | Liq. |
| 14 |

|

|
2 | 96 | 103–106 | 104–105 |
| 15 |

|

|
2 | 95 | Liq. | Liq. |
| 16 |

|

|
3 | 92 | Liq. | Liq. |
Optimal conditions: 1 mg of CCPSF NPs and power of microwave irradiation 500 (W).
