Abstract
Nail-patella syndrome (NPS) is a rare autosomal dominant disease characterized by nail dysplasia, aplastic or hypoplastic patellae, elbow dysplasia, and presence of iliac horns. Renal or ocular abnormalities are also associated with the disease. We report the case of a 57-year-old woman affected by NPS and having haploinsufficiency of the LMX1B gene who experienced severe bilateral chronic angle-closure glaucoma in both eyes and that was successfully managed with a flap-express procedure in the right eye. The left eye had no light perception, and medical treatment was considered. Glaucoma is the most frequent ocular abnormalities observed in association with NPS and usually presents with an open angle. Glaucoma associated with NPS typically has an early onset open-angle phenotype. In fewer cases, it may present with an angle-closure phenotype. Therefore, we emphasize the need for glaucoma case-finding protocols comprehensive of gonioscopy in NPS patients and their relatives.
Keywords: Nail-patella syndrome, Glaucoma, LMX1B, Filtering surgery
Introduction
Nail-patella syndrome (NPS) is an autosomal dominant disorder characterized by a classical clinical tetrad involving nail dysplasia, aplastic or hypoplastic patellae, elbow dysplasia, and the presence of iliac horns [1]. Besides nails and osteoarticular defects, a significant proportion of patients present with multisystemic involvement, namely, renal anomalies extending from asymptomatic proteinuria to end-stage renal failure [2]. Among other involved organs, ocular anomalies and ocular sight-threatening diseases have been consistently reported in patients affected by NPS [1, 3–10]. NPS was first described in the 1800s, and more recently, in 1998, the LMX1B mutations (9q33.3) leading to loss of function of the LMX1B protein were identified as responsible for NPS [11]. LMX1B gene encodes a transcription factor that belongs to the LIM-homeodomain family of proteins. These proteins are essential for the normal development of dorsal limb structures in vertebrates, the renal glomerular filtration barrier components, and the anterior segment of the eye [2, 12]. Therefore, the haploinsufficiency of this transcription factor has been implicated in the pathogenesis of NPS multisystemic disease. Ocular anomalies have been initially described among people affected by NPS, with phenotypes varying from ocular hypertension (OHT) to pigmentary glaucoma and bilateral congenital glaucoma [3]. In cases where glaucoma was clinically indistinguishable from typical primary open-angle glaucoma (OAG), a relatively young age at diagnosis (median: 38, interquartile range 30–43 years) was observed [3, 11]. Of note, no manifest gonioscopic signs of angle dysgenesis were observed in these cases. In other studies on NPS patients, glaucoma or OHT was found in 20–33% of patients aged 40 years or more, significantly more than the prevalence expected in an age-matched population without NPS [1, 4, 7]. Other ocular abnormalities reported in NPS patients included microcornea, sclerocornea, congenital cataract, and the presence of the Lester’s sign [2, 13]. Lester’s sign consists of a zone of darker pigmentation around the central part of the iris, but this sign is not specific since it is also frequently observed in the normal population [1, 13]. Recently, a case of NPS associated with bilateral symptomatic angle closure caused by plateau syndrome was described [10]. We report another case of NPS associated with angle-closure glaucoma.
Case Report
A 57-year-old woman of African descent was referred in February 2021 to the glaucoma unit of Clinica Oculistica San Martino Polyclinic Hospital, Genoa, Italy, to manage a medically uncontrolled bilateral glaucoma. At the first evaluation, the patient complained about progressively decreased vision in both eyes. Carefully interviewed, she reported a history of glaucoma for about 5 years, currently on therapy with brinzolamide/timolol b.i.d. (Azarga, Novartis Farma SpA, Italy) and latanoprost q.d. (Xalatan, Medifarm Srl, Italy). Best-corrected visual acuity was logMAR 0.5 in the right eye and no light perception in the left eye. In the right and left eyes, the intraocular pressure (IOP) was 48 mm Hg and 60 mm Hg, respectively.
Besides a subtle microcystic corneal oedema in the left eye, the slit-lamp examination revealed a within anterior limit anterior segment and a trace of lens nuclear sclerosis for both eyes. Furthermore, corneal dynamic gonioscopy showed bilateral peripheral anterior synechiae extending almost entirely over the circumference. In addition, the neuroretinal rim of the right optic disk was very thin, whereas the left eye papilla was atrophic. Then, acetazolamide 250 mg per os q.i.d was prescribed, and gentle decompression was performed, via an inferotemporal paracentesis, in both eyes. The day after, IOP decreased to 18 and 30 mm Hg in the right and left eyes, respectively. Despite maximum tolerated medical therapy, IOP remained above the target, and a surgical procedure was scheduled for the right eye. Trabeculectomy with Ex-PRESS implantation was performed, and after a year of follow-up, IOP was stable in the mid-teens (Figs. 1, 2).
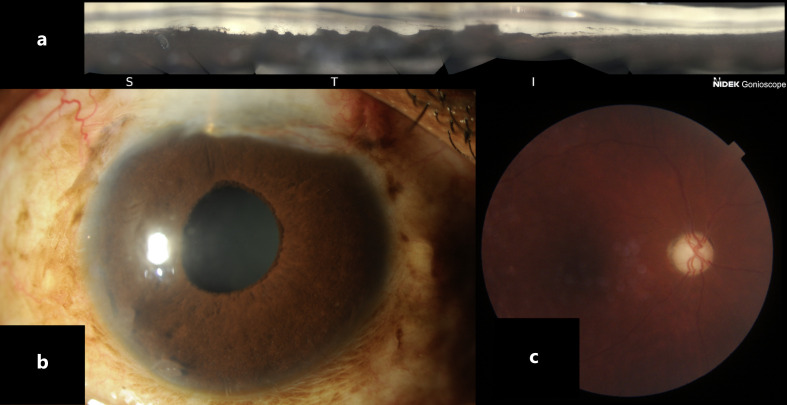
Fig. 1.
Right eye: gonioscopic view (a), slit-lamp photo (b), fundus photo (c).
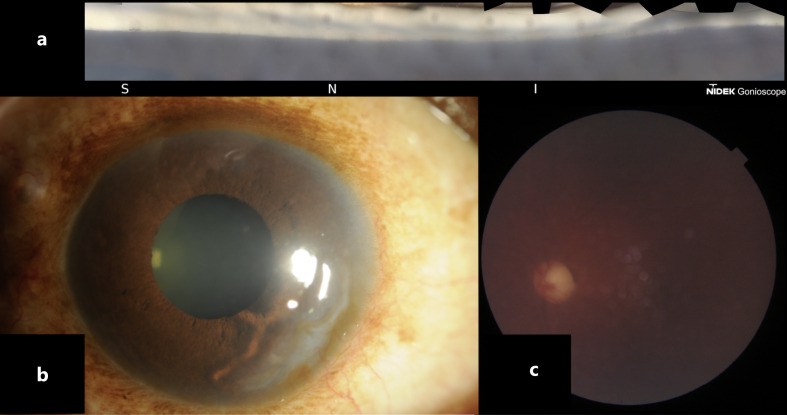
Fig. 2.
Left eye: gonioscopic view (a), slit-lamp photo (b), fundus photo (c).
Besides the glaucoma diagnosis, the patient was diagnosed in 1990, at the age of 26 years old with NPS based on clinical criteria (Fig. 3). No renal involvement was noted during the course of the disease, and glaucoma was not investigated at the time of NPS diagnosis. Later, in 2016 genetic analysis confirmed the diagnosis of NPS, revealing haploinsufficiency of the LMX1B gene (c.312dup> [p.Gln105Thr/s*43]), described in the literature as causative of the NPS. Clinical features of NPS were hypoplastic nails with absent lunulae and loss of distal interphalangeal skin creases (Fig. 1), and bilateral patellar hypoplasia (Fig. 2). She reported the diagnosis of NPS also in her only daughter with mild renal involvement and no ocular disease. Unfortunately, no access to detailed medical records was possible.
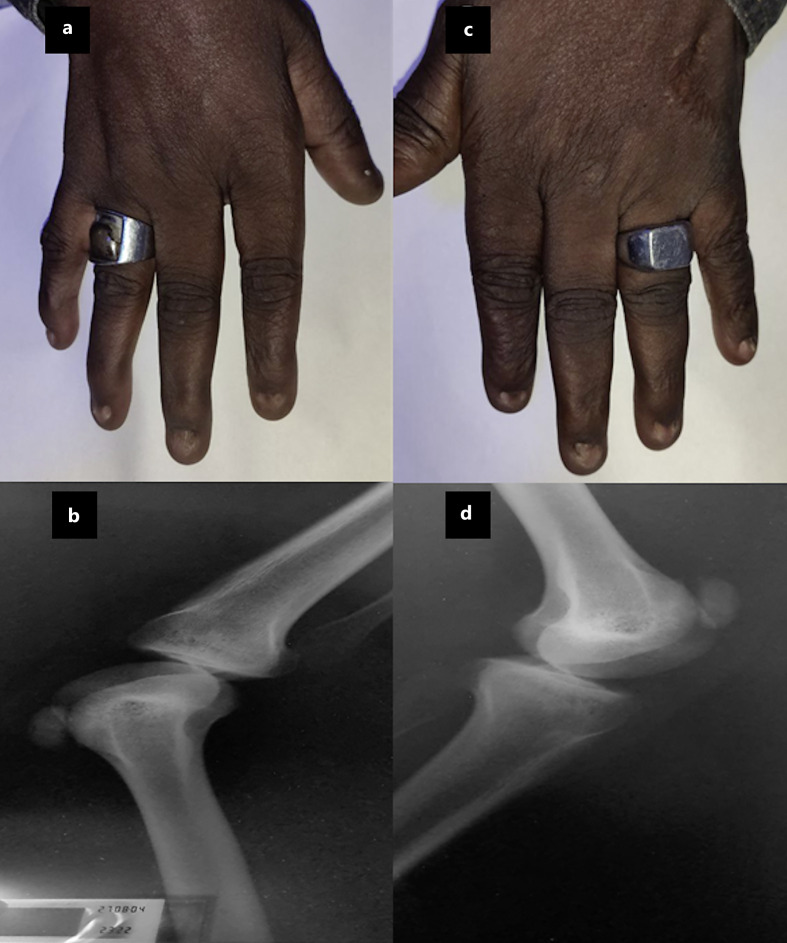
Fig. 3.
Clinical features of nail-patella syndrome. Hypoplastic nails with absent lunulae and loss of distal interphalangeal skin creases on fingers of right hand (a) and left hand (c). X-ray scan of the knees showing a bilateral hypoplastic patella in right (b) and left leg (d).
Discussion
Since the initial description of NPS more than a century ago, several involved organ anomalies have been associated with the condition in the last two decades [7, 13, 14]. Renal and ocular involvements are the most severe comorbidities related to NPS and may represent a significant part of the disease burden [4, 14]. Our work presented a case of bilateral chronic angle-closure glaucoma unresponsive to medical treatment and treated by a filtering procedure. Previous studies have described a broad spectrum of glaucomas or OHT associated with NPS encompassing congenital glaucoma, pigmentary glaucoma, OAG, normal-pressure glaucoma, and plateau iris syndrome [3, 5, 6, 10]. Table 1 summarizes the studies reporting association between glaucoma and NPS. Even if glaucoma is a relatively common eye disease and its co-existence with other ocular or systemic diseases is not surprising, several clues link NPS and glaucoma pathogenesis. First, since the early reports by Litcher et al. [3], the onset of glaucoma in NPS patients was observed at a younger age than what is expected with primary OAG. Even if the gonioscopic features of NPS glaucoma patients described by Litcher were not suggestive of angle dysgenesis, nowadays, optical coherence tomography scans of the anterior chamber angle have shown abnormalities in the trabecular meshwork and Schlemm’s canal that are otherwise undetected by standard angle examination techniques [15]. Thus, it is impossible to rule out that some form of angle dysgenesis could exist even in a normal-appearing angle.
Table 1.
Summary of studies reporting the association between glaucoma and NPS
| First author (year) | Study design | NPS cases, n | Glaucoma diagnosis, n | Type of glaucoma | Age of diagnosis |
|---|---|---|---|---|---|
| Lichter et al. [3] (1997) | Case series | Family 1: 13 Family 2: 11 |
6 7 |
GL: 6 GL: 5 PG: 1 PDS: 1 |
Mean age family 1: 32 yo Mean age family 2: 24 yo (2 cases of congenital glaucoma) |
| Fröhlich et al. [9] (2002) | Case report | 2 | 1 | OAG | 42 |
| Sweeney [1] (2003) | Case series | 83 | 14 | GL: 8 OHT: 6 |
Mean age: 47.9 years (23–78 years) |
| Bongers [2] (2005) | Case series | 51 | 17 | OAG: 2 NTG: 4 OHT: 2 GL: 9 |
Mean age: 63.4 yo 1 case of OAG <40 yo 1 case of OHT <40 yo |
| Mimiwati et al. [4] (2006) | Case series | 19 | 4 | OAG: 1 NTG: 1 OHT: 2 |
33 63 14–24 |
| Millá et al. [5] (2017) | Case series | 10 | 7 | OAG: 1 OHT: 6 |
|
| Romero et al. [6] (2011) | Case series | 5 | 4 | GL: 3 OHT: 1 |
57–53–42 21 |
| Ghoumid et al. [7] (2016) | Case series | 43 | 9 | GL: 9 | 5 of them <40 yo |
| Nicolle et al. [8] (2017) | Case report | 1 | 1 | OAG | About 35 yo |
| Gardin et al. [10] (2020) | Case report | 1 | 1 | Plateau iris syndrome and angle-closure glaucoma | 32 yo |
| Total | 239 (only case series) | 71 (29.7%) |
Only cases reporting an eye examination were included. GL, glaucoma; NL, normal; NPS, nail-patella syndrome; OHT, ocular hypertension; NTG, normal tension glaucoma; OAG, open-angle glaucoma; PG, pigmentary glaucoma; PDS, pigment dispersion syndrome; IOP, intraocular pressure; yo, years old.
The second clue that links NPS with glaucoma is represented by the protein coded by the role LMX1B gene, whose loss-of-function mutations cause NPS [11]. LIM homeobox transcription factor 1-beta is essential for the normal development of dorsal limb structures, the glomerular basement membrane, dopaminergic and serotonergic neurons, and the anterior segment of the eye [11]. Animal studies have shown that homozygous for a targeted mutation of LMX1B exhibits iris and ciliary body, hypoplasia, and cornea stromal abnormalities [16]. Moreover, by inducing different LMX1B mutations in mice, further research has demonstrated that different LMX1B mutations can result in elevated IOP and glaucomatous damage. LMX1B-mutated mice represent a rare animal model of human glaucoma caused by mutation of the same gene in humans and mice [17].
Lastly, a case-control genetic association study has shown that specific LMX1B haplotypes influence susceptibility to glaucoma in the general population and a genome-wide analyses also identify that some SNPs significantly associated with IOP in healthy population annotate near LMX1B gene [18, 19]. Glaucoma is a progressive neurodegenerative eye condition caused by retinal ganglion cell apoptosis where IOP is the major modifiable risk factor for its onset and progression [20]. If untreated, glaucoma may lead to severe visual disability and irreversible blindness. Abnormalities in the anterior segment, particularly in the irido-corneal angle of the eye, may cause impaired aqueous humor reabsorption by the trabecular meshwork and hence IOP elevation. Since glaucoma is generally asymptomatic, progressive, and irreversible, early diagnosis and effective treatment by reducing IOP are of paramount importance. Whereas screening for glaucoma in the general population is not considered cost-effective, it is recommended in NPS because of the high prevalence of their association, early onset of the disease, and relatively few patients affected by NPS to be submitted for an ophthalmological examination [21].
Conclusion
Our study highlights the importance of screening for glaucoma comprehensive of gonioscopy in NPS patients. Moreover, we speculate that angle examination with modern technologies such as optical coherence tomography may show abnormalities of the trabecular meshwork or Schlemm’s canal even in NPS patients with normal-appearing angles. This hypothesis should be tested in further studies.
Statement of Ethics
Ethics approval was not required in accordance with local or national guidelines. Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images. This report does not contain any personal information that could lead to the identification of the patient.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There were no funding sources for this case report.
Author Contributions
Nicola Pallozzi Lavorante, Michele Iester, Chiara Bonzano, Alessandro Bagnis, Carlo Enrico Traverso, and Carlo Alberto Cutolo: substantial contribution to conception and design of the case report and drafting the manuscript and gave final approval to be published.
Funding Statement
There were no funding sources for this case report.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1. Sweeney E. Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet. 2003 Mar;40(3):153–62. 10.1136/jmg.40.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bongers E, Gubler M-C, Knoers N. Nail-patella syndrome. Overview on clinical and molecular findings. Pediatr Nephrol. 2002 Sep;17(9):703–12. 10.1007/s00467-002-0911-5. [DOI] [PubMed] [Google Scholar]
- 3. Lichter PR, Richards JE, Downs CA, Stringham HM, Boehnke M, Farley FA. Cosegregation of open-angle glaucoma and the nail-patella syndrome. Am J Ophthalmol. 1997 Oct;124(4):506–15. 10.1016/s0002-9394(14)70866-9. [DOI] [PubMed] [Google Scholar]
- 4. Mimiwati Z, Mackey DA, Craig JE, Mackinnon JR, Rait JL, Liebelt JE, et al. Nail-patella syndrome and its association with glaucoma: a review of eight families. Br J Ophthalmol. 2006 Dec;90(12):1505–9. 10.1136/bjo.2006.092619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Millá E, Hernan I, Gamundi MJ, Martínez-Gimeno M, Carballo M. Novel LMX1B mutation in familial nail-patella syndrome with variable expression of open angle glaucoma. Mol Vis. 2007 Apr;13:639–48. [PMC free article] [PubMed] [Google Scholar]
- 6. Romero P, Sanhueza F, Lopez P, Reyes L, Herrera L. c.194 A>C (Q65P) mutation in the LMX1B gene in patients with nail-patella syndrome associated with glaucoma. Mol Vis. 2011;17:1929–39. [PMC free article] [PubMed] [Google Scholar]
- 7. Ghoumid J, Petit F, Holder-Espinasse M, Jourdain A-S, Guerra J, Dieux-Coeslier A, et al. Nail-Patella Syndrome: clinical and molecular data in 55 families raising the hypothesis of a genetic heterogeneity. Eur J Hum Genet. 2016 Jan;24(1):44–50. 10.1038/ejhg.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nicolle P, Baudouin C, Brasnu E. [Nail-patella syndrome and glaucoma: a case report]. J Fr Ophtalmol. 2017 Feb;40(2):e51–3. 10.1016/j.jfo.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 9. Fröhlich SJ, Kalpadakis P, Rudolph G, Boergen KP. [Ocular involvement in nail-patella syndrome (#161200)]. Ophthalmologe. 2002 Apr;99(4):281–5. 10.1007/s003470100553. [DOI] [PubMed] [Google Scholar]
- 10. Gardin MA, Khor CC, Silva L, Krefting EA, Ritch R. Plateau iris syndrome and angle-closure glaucoma in a patient with nail-patella syndrome. Am J Ophthalmol Case Rep. 2020 Aug;20:100886. 10.1016/j.ajoc.2020.100886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vollrath D, Jaramillo-Babb VL, Clough MV, McIntosh I, Scott KM, Lichter PR, et al. Loss-of-Function mutations in the LIM-homeodomain gene, LMX1B, in nail-patella syndrome. Hum Mol Genet. 1998 Jul;7(7):1091–8. 10.1093/hmg/7.7.1091. [DOI] [PubMed] [Google Scholar]
- 12. Tolman NG, Balasubramanian R, Macalinao DG, Kearney AL, MacNicoll KH, Montgomery CL, et al. Genetic background modifies vulnerability to glaucoma-related phenotypes in Lmx1b mutant mice. Dis Model Mech. 2021 Feb;14(2):dmm046953. 10.1242/dmm.046953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flickinger RR, Spivey BE. Lester’s line in hereditary osteo-onychodysplasia. Arch Ophthalmol. 1969 Nov;82(5):700–3. 10.1001/archopht.1969.00990020694020. [DOI] [PubMed] [Google Scholar]
- 14. Lemley KV. Kidney disease in nail-patella syndrome. Pediatr Nephrol. 2009 Dec;24(12):2345–54. 10.1007/s00467-008-0836-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Varshney T, Azmira K, Gupta S, Mahalingam K, Singh A, Angmo D, et al. In vivo imaging of the Schlemm’s canal and the response to selective laser trabeculoplasty. Am J Ophthalmol. 2022 Feb;234:126–37. 10.1016/j.ajo.2021.07.002. [DOI] [PubMed] [Google Scholar]
- 16. Pressman CL, Chen H, Johnson RL. LMX1B, a LIM homeodomain class transcription factor, is necessary for normal development of multiple tissues in the anterior segment of the murine eye. Genesis. 2000 Jan;26(1):15–25. . [DOI] [PubMed] [Google Scholar]
- 17. Cross SH, Macalinao DG, McKie L, Rose L, Kearney AL, Rainger J, et al. A dominant-negative mutation of mouse Lmx1b causes glaucoma and is semi-lethal via LBD1-mediated dimerisation. PLoS Genet. 2014 May;10(5):e1004359. 10.1371/journal.pgen.1004359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park S, Jamshidi Y, Vaideanu D, Bitner-Glindzicz M, Fraser S, Sowden JC. Genetic risk for primary open-angle glaucoma determined by LMX1B haplotypes. Invest Ophthalmol Vis Sci. 2009 Apr;50(4):1522–30. 10.1167/iovs.08-2483. [DOI] [PubMed] [Google Scholar]
- 19. Khawaja AP, Cooke Bailey JN, Wareham NJ, Scott RA, Simcoe M, Igo RP Jr, et al. Genome-wide analyses identify 68 new loci associated with intraocular pressure and improve risk prediction for primary open-angle glaucoma. Nat Genet. 2018 Jun;50(6):778–82. 10.1038/s41588-018-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. European Glaucoma Society terminology and guidelines for glaucoma, 5th edition. Br J Ophthalmol. 2021 Jun;105(Suppl 1):1–162. [DOI] [PubMed] [Google Scholar]
- 21. Galloway G, Vivian A. An ophthalmic screening protocol for nail-patella syndrome. J Pediatr Ophthalmol Strabismus. 2003 Jan;40(1):51–3. 10.3928/0191-3913-20030101-15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.