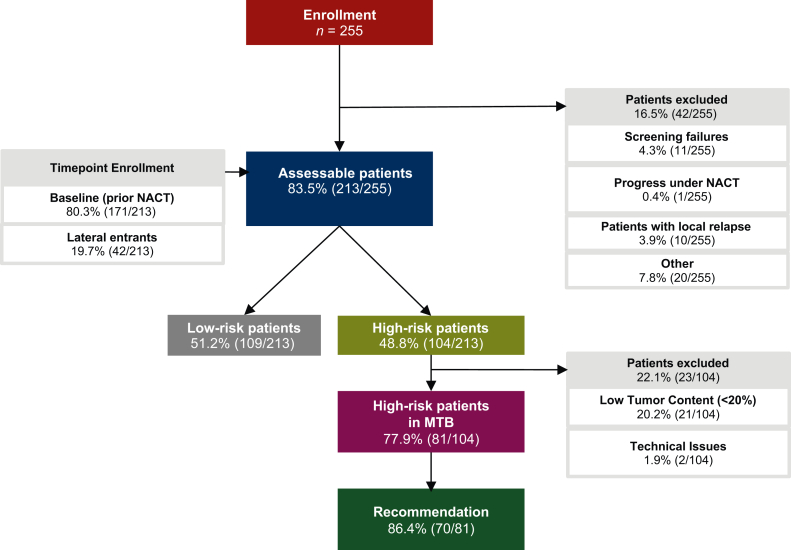
Figure 1.
Cohort characteristics. Flow diagram displaying the number of patients enrolled, assessable, and excluded from analysis. Patients were conventionally enrolled before start of neoadjuvant chemotherapy (NACT; baseline patients), but also after the start of the course of NACT (lateral entrants). Risk status for relapse after NACT based on the pathological evaluation was assessed in a standard-of-care post-operative tumor board. In high-risk patients molecular profiling (whole-genome or whole-exome as well as transcriptome sequencing) was carried out and patients were presented in an interdisciplinary molecular tumor board (MTB) to prioritize molecular-guided therapies. A total of 213 assessable patients were distributed among immunohistochemistry-based subtypes as follows: 75 HR+HER2–, 39 HR+HER2+, 19 HER2+HR–, and 80 TNBC cases. HER2, human epidermal growth factor receptor 2; HR, hormone receptor; TNBC, triple-negative breast cancer.