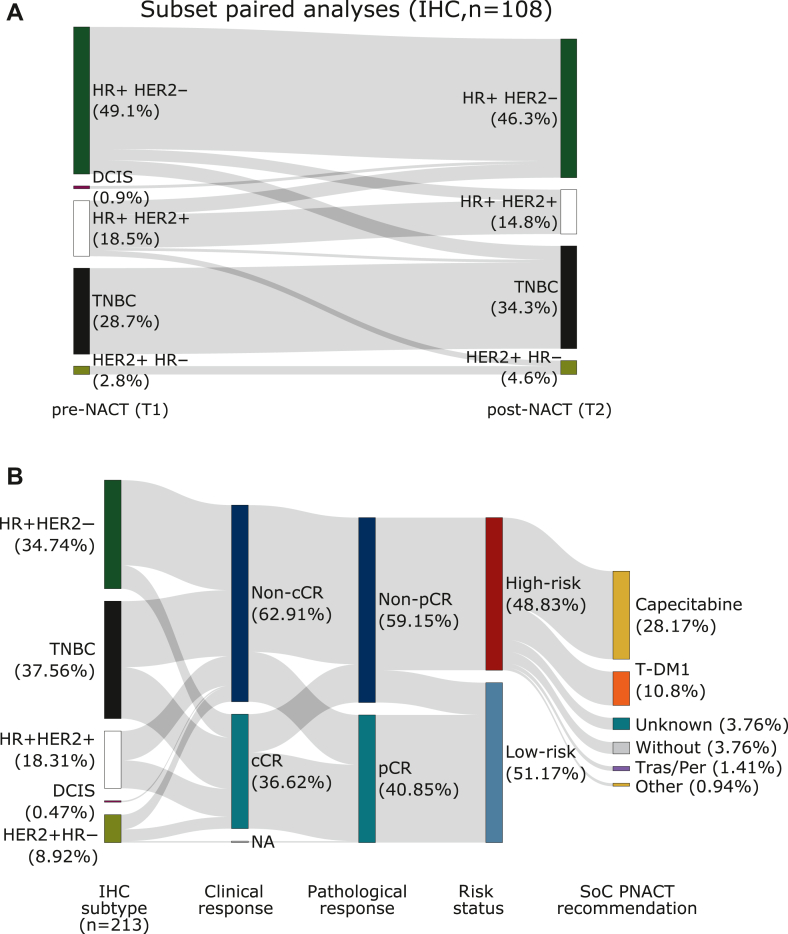
Figure 2.
Pre- and postdynamics of subtype and prognostic risk status. (A) Sankey plot showing the distribution of paired subtypes before and after neoadjuvant chemotherapy (NACT; n = 108) determined by immunohistochemistry (IHC) for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). One patient displayed a ductal carcinoma in situ, which is conventionally not classified according to the standard IHC-based subtyping. Based on the assessment of the overall risk status, 104 patients possessed a high risk, yet 108 patients were eligible for the IHC-based paired subtyping. (B) Analysis of image-based (ultrasound, mammography, and/or magnetic resonance imaging) clinical response, classified as complete clinical response (cCR) or noncomplete clinical response (non-cCR), and its relation to the immunohistochemistry (IHC)-based subtyping, the pathology-based pathological response, classified as complete pathological response (pCR) or non-pCR, and the respective prognostic risk status. In one patient clinical response was not evaluable. Post-neoadjuvant treatment recommendations in the standard of care (SoC) include capecitabine, T-DM1, trastuzumab/pertuzumab (Tras/Per), and trastuzumab/pertuzumab/capecitabine. For 7.7% of the patients, the therapy protocol was unknown and 7.7% of the patients did not receive any post-neoadjuvant therapy (PNACT) because of intolerability of NACT and comorbidities. DCIS, ductal carcinoma in situ; HR, hormone receptor; NA, missing data; TNBC, triple-negative breast cancer.