Abstract
Background
While early mobilization is commonly implemented in intensive care unit treatment guidelines to improve functional outcome, the characterization of the optimal individual dosage (frequency, level or duration) remains unclear. The aim of this study was to demonstrate that artificial intelligence-based clustering of a large ICU cohort can provide individualized mobilization recommendations that have a positive impact on the likelihood of being discharged home.
Methods
This study is an analysis of a prospective observational database of two interdisciplinary intensive care units in Munich, Germany. Dosage of mobilization is determined by sessions per day, mean duration, early mobilization as well as average and maximum level achieved. A k-means cluster analysis was conducted including collected parameters at ICU admission to generate clinically definable clusters.
Results
Between April 2017 and May 2019, 948 patients were included. Four different clusters were identified, comprising “Young Trauma,” “Severely ill & Frail,” “Old non-frail” and “Middle-aged” patients. Early mobilization (< 72 h) was the most important factor to be discharged home in “Young Trauma” patients (ORadj 10.0 [2.8 to 44.0], p < 0.001). In the cluster of “Middle-aged” patients, the likelihood to be discharged home increased with each mobilization level, to a maximum 24-fold increased likelihood for ambulating (ORadj 24.0 [7.4 to 86.1], p < 0.001). The likelihood increased significantly when standing or ambulating was achieved in the older, non-frail cluster (ORadj 4.7 [1.2 to 23.2], p = 0.035 and ORadj 8.1 [1.8 to 45.8], p = 0.010).
Conclusions
An artificial intelligence-based learning approach was able to divide a heterogeneous critical care cohort into four clusters, which differed significantly in their clinical characteristics and in their mobilization parameters. Depending on the cluster, different mobilization strategies supported the likelihood of being discharged home enabling an individualized and resource-optimized mobilization approach.
Trial Registration: Clinical Trials NCT03666286, retrospectively registered 04 September 2018.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-022-04291-8.
Keywords: Early ambulation, Critical care, Critical illness, Physical therapy modalities, Patient discharge
Introduction
Early mobilization has been suggested as a promising intervention to counteract intensive care unit acquired weakness (ICUAW) by attenuating the muscle wasting associated with critical illness [1]. Previous clinical studies with an early intervention start have shown, early mobilization can be safely initiated in the ICU and might improve the functional capacity, reduce days with mechanical ventilation in the ICU and increase the rate of discharge home [1–5]. However, in the recently published TEAM trial increasing active early mobilization did not result in significantly reduced hospital length of stay or mortality compared to usual ICU mobilization. In addition, the rate of adverse events was increased in the intervention group [6]. This raises the question which patients benefit most from early mobilization and how to determine the appropriate type, timing, intensity, coordination and duration of therapy [7]. Wide variability was found in intervention characteristics, outcome measures and associated metrics, leading to conflicting results regarding the influence of early mobilization dosage on functional status after ICU stay [8, 9]. Commonly, only the maximum level of mobilization achieved is quantified, whereas the duration, intensity and frequency as important parameters of the dosage of mobilization are not [10]. Since the group of intensive care patients is extremely heterogeneous, it is difficult to recommend interventions across all patients. The relationship between severity of illness, age, weight and the presence of functional impairment and comorbidities regarding the implementation of rehabilitation also remains unclear [8, 11–13]. As a result, it is difficult to determine the appropriate timing and dose of intervention to achieve the optimal benefit for the respective patient [9, 14, 15].
An individualized approach to early mobilization which considers the pre-existing functional status, frailty, comorbidities, disease severity and invasiveness of the treatment in the ICU might be meaningful. The aim of this study was to test that it is feasible to divide the diverse group of ICU patients into specific cohorts by clustering and derive specific individualized recommendations for mobilization to increase the probability to be discharged home. We hypothesized that identifiable patient groups benefit differently from different mobilization components.
Methods & materials
Study design, setting and participants
This study is an analysis of our prospective observational database of two interdisciplinary intensive care units of the Department of Anaesthesiology and Intensive Care at Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany between April 2017 and May 2019. The database is registered at Clinical Trials (NCT03666286, registered 04 September 2018) and approved by the Ethics Committee of the Faculty of Medicine, Technical University of Munich (518/16S). Adults with > 24 h stay in the ICU were included in the database, if consent was obtained either by the patient or legal representative according to German legislation. Patients were included in the analysis, whose mobilization during the intensive care unit stay was fully recorded.
Outcome variables
The primary outcome was discharge disposition “home.” It was tested against the combination of all adverse discharge dispositions (nursing home, hospice, another hospital or death), considering it as the optimal outcome after critical illness as opposed to institutionalization [16, 17]. Secondary outcome variables are ICU mortality, hospital mortality, ICU length of stay (LOS) and hospital LOS.
Factors of interest
The factor of interest was mobilization using the surgical ICU optimal mobilization score (SOMS) (representing active and passive mobilization) [18–20] and the ICU Mobility Scale (IMS) (representing only active mobilization) [21–23]. The SOMS describes a patient’s mobilization capacity on a numerical rating scale ranging from 0 (no mobilization) to 4 (ambulation) capturing both active and passive mobilization forms [4]. The IMS captures active forms of mobilization and ranges from 0 “lying in bed” to 10 “walking independently without a gait aid” with an excellent inter-rater reliability if used in critically ill patients [21]. To determine the dosage of mobilization, sessions per day, mean duration per day (in minutes), first day of mobilization, average and maximum level reached during ICU stay and the distribution of the individual levels achieved were recorded. Mobilization was defined as “early” if it occurred within the first 72 h after intensive care admission [24, 25]. Mobilization could be performed by all professionals working in the ICU and not exclusively by physiotherapists.
Data collection
Data included upon admission were basic demographics, location prior to ICU admission, ICU admission category (sepsis, polytrauma, traumatic brain injury, non-traumatic brain injury, postoperative monitoring, cardiac failure, respiratory failure and “other”) and diagnosis (e.g., sepsis or trauma) and several scores to characterize the cohort: baseline Glasgow Coma Scale (GCS), Clinical Frailty Scale (CFS) [26, 27], Charlson Comorbidity Index [28], Sequential Organ Failure Assessment score (SOFA) [29] as well as standard laboratory and hemodynamic parameters. Functional status was assessed using two relevant sub-domains of the Barthel Index, an ordinal scale comprising ten sub-domains of activities of daily living, which is the most used scale for activities of daily living [30, 31]. The scores of the “mobility” and “transfer” sub-domains of the Barthel (“Mobility-Transfer-Barthel,” MTB) index represent a patient’s functional ability and gait independence with a minimum of 0 points (functionally totally dependent) and a maximum of 30 points (functionally independent) [32]. A premorbid baseline value was obtained representing the functional status two weeks before hospital admission. Upon ICU discharge, data was obtained regarding ICU LOS and mortality, as well as data about ICU-related therapy (e.g., fluid administration, nutrition, dialysis and laboratory parameters). Upon hospital discharge, data on LOS and discharge disposition (prior residence, nursing home, rehabilitation clinic, etc.) as well as mortality was obtained. The compilation of these variables led to a comprehensive characterization of our cohort in terms of the feasibility of mobilization. In addition to patient characteristics (age, Body Mass Index, sex), functional status before the critical illness (frailty, Mobility-Transfer-Barthel, Charlson Comorbidity Index) as well as disease severity (SOFA, APACHE II, Glasgow Coma Scale) and condition at ICU admission were recorded in detail.
Statistical analysis
Clustering
Since the underlying patient collective was a very inhomogeneous cohort, an attempt was made to find groups that were as similar as possible and as different from each other as possible. To achieve this goal, a k-means cluster analysis was conducted using the method k-means from the base R with the following factors: sex, age, Body Mass Index, Mobility-Transfer-Barthel at hospital admission, department (e.g., neurosurgery, cardiology), admission form (e.g., from home, another hospital, nursing home), Clinical Frailty Scale, Glasgow Coma Scale, APACHE 2 score, SOFA score, Charlson Comorbidity Index, and ICU admission categories (sepsis, polytrauma, traumatic brain injury, non-traumatic brain injury, postoperative monitoring, cardiac failure, respiratory failure and “other”). A crucial part of the k-means cluster analysis is the selection of k, which represents the number of groups to be identified by the clustering algorithm. We chose the elbow method to determine the optimal number of clusters. Since the cluster analysis is an unsupervised method, the resulting clusters must be named according to the characteristics of the included expressions [33]. Cluster stability assessment was checked using the average Jaccard index on 1000 bootstrap samples [34]. To delineate the performance of the clustering method, Euclidean distances were calculated and plotted [34]. To visualize clustering results, a Principal Component Analysis plot for k-means clustering using the first two components was plotted.
Analysis
In each cluster, the influence of the mobilization parameters on the primary endpoint “discharge home” for all patients including patients who died, was analyzed. The influence of the four mobilization parameters on the primary endpoint was analyzed using Mann–Whitney U tests or Fisher's exact tests. Then, a logistic regression model using only the four mobilization parameters was calculated. For model adjustments, stepwise forward logistic regression models were computed. In these models, all mobilization parameters were mandatory to stay in the model and all variables used for clustering were added in a stepwise forward manner, using Akaike Information Criterion as a means of model performance.
The mobilization parameters were also compared between the clusters using Kruskal–Wallis test for continuous variables and Fishers exact test for categorical variables. Post hoc analysis was conducted using Mann–Whitey U tests and Fishers exact tests with Bonferroni correction.
All continuous variables are presented as median with interquartile range [IQR], all categorical variables with absolute and relative numbers. An alpha of 5% was selected as level of significance. We further performed several sensitivity analyses: First, the primary analysis was repeated in survivors only in all patients and the four clusters. Secondly, the primary analysis was repeated with the 11-point IMS Score. All analyses were performed using R version 4.2.1 (R Foundation for Statistical Computing; Vienna Austria).
Power analysis
According to Vittinghoff and McCulloch, a case number of 5–9 patients per variable with the smaller outcome category is required to ensure sufficient power [35]. With the four mobilization parameters included in the analysis, we had to ensure that there were at least 20 patients with the smaller outcome category in the cluster.
Missing values
Variables with more than 5% missing values were excluded from the study. For continuous variables, median imputation was performed. For categorical variables, a category for the missingness was added.
Results
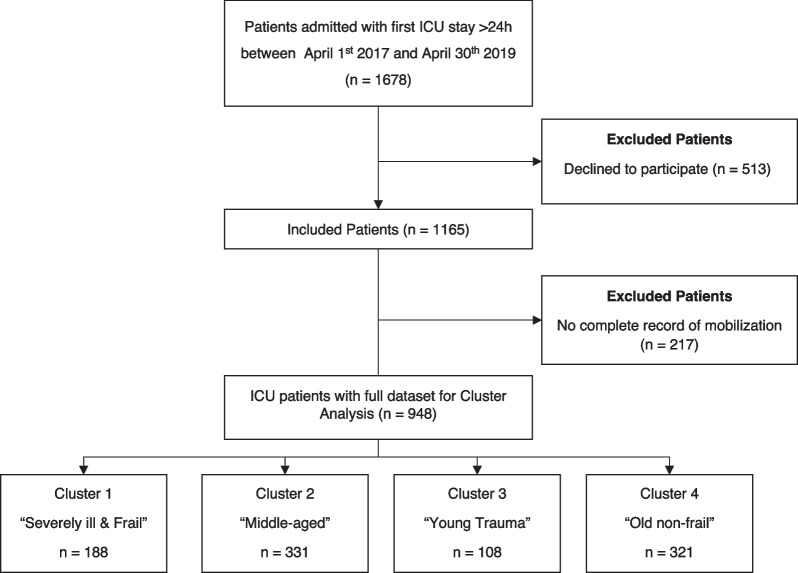
Between April 1st, 2017 and May 31st, 2019, we included 948 patients (Fig. 1). The characteristics of the 300 patients (32%) who could be discharged home differed significantly from all others (see Additional file 1: Table S1).
Fig. 1.
STROBE Diagram
Cluster analysis
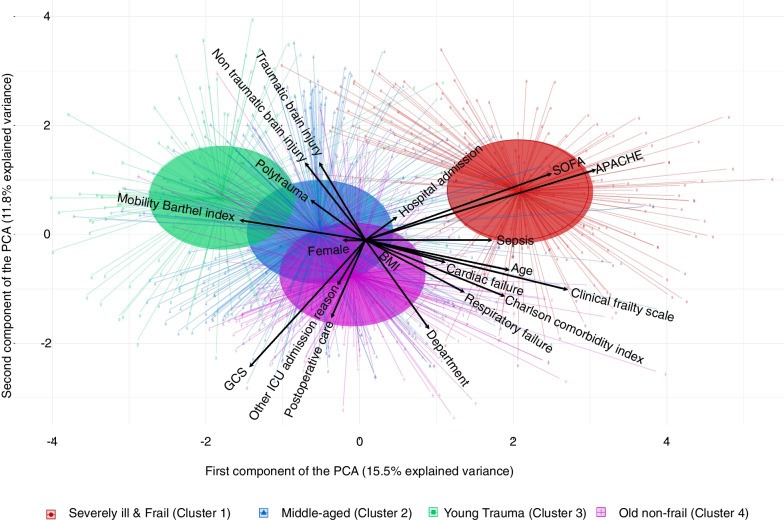
No variable had a rate of missing data over 5%. Missing data from BMI, APACHE II and SOFA score had to be imputed with the median. The elbow method showed an optimal number of four clusters (Additional file 1: Figure S1). Each cluster contained clinically determinable patient characteristics and was represented in the Principal Component Analysis plot for k-means clustering (Fig. 2): Clusters were labeled according to the major loads resulting in clusters “Severely ill & Frail,” “Young Trauma,” “Old non-frail” and “Middle-aged”. The patients differed between the clusters in terms of several characteristics (Table 1). Post hoc analyses are presented in Additional file 1: Table S2. Jaccard Index ranged from 0.74 in cluster “Severely ill & Frail” patients to 0.94 in cluster “Young Trauma”. The Euclidean distance plot can be found in the Additional file 1: Figure S2.
Fig. 2.
Biplot of the Cluster centers on the first two dimensions of a Principal Components Analysis (PCA). Arrows illustrate the strength and direction of the influence of the variables on the first and the second component of the PCA. The higher the value of a variable, the longer the arrow, and the stronger the influence in the direction of the arrow. The colored ellipses show the cluster centers. The dots in different colors indicate individual patients and their belonging to the clusters. First component of the PCA explains 15.5% of the variance in the data and is highly positively loaded with APACHE, SOFA and frailty scores and highly negatively loaded with Mobility-Transfer-Barthel, GCS, as well as the admission reasons non-traumatic brain injury, tic brain injury and polytrauma. The second component explains 11.8% of the variance in the data and is highly positive loaded with APACHE, non-traumatic brain injury and traumatic brain injury and highly negative loaded with GCS, department and the admission reason postoperative. The red cluster is mainly loaded with high APACHE, SOFA and Clinical Frailty Scale, which is why it is labeled “Severely ill & Frail”. The green cluster is mainly loaded with young age, high Mobility-Transfer-Barthel, and polytrauma, which is why it is labeled “Young Trauma”. The purple cluster is mainly loaded with allocation for postoperative treatment due to old age but low SOFA, APACHE and Clinical Frailty Scale, which is why it is labeled “Old non-frail”. The blue cluster has no specific load from the first or second principal component. Since the cluster’s median age is close to that of the total cohort, it is labeled “Middle-aged”. GCS Glasgow Coma Scale, SOFA Sepsis-related organ failure assessment score, APACHE Acute physiology and chronic health evaluation score
Table 1.
Patient characteristics in the four clusters
| Severely ill & Frail | Middle-aged | Young Trauma | Old non-frail | P value | |
|---|---|---|---|---|---|
| (N = 188) | (N = 331) | (N = 108) | (N = 321) | ||
| Female sex | 107 (56.9) | 202 (61.0) | 60 (55.6) | 185 (57.6) | 0.67 |
| Body Mass Index (kg/m2) | 25.4 [23.5–27.7] | 25.7 [23.1–28.1] | 24.2 [21.1–26.7] | 25.4 [23.3–28.1] | 0.007 |
| Underweight | 5 (2.7) | 21 (6.3) | 7 (6.5) | 14 (4.4) | 0.52 |
| Normal | 74 (39.4) | 123 (37.2) | 60 (55.6) | 132 (41.1) | |
| Overweight | 82 (43.6) | 135 (40.8) | 30 (27.8) | 130 (40.5) | |
| Obese | 27 (14.4) | 52 (15.7) | 11 (10.2) | 45 (14.0) | |
| Age (years) | 78 (73–82) | 59 (54–63) | 34 (27–40) | 75 (71–80) | < 0.001 |
| ≤ 50 | 0 (0.0) | 46 (13.9) | 108 (100.0) | 0 (0.0) | < 0.001 |
| 51–65 | 11 (5.9) | 268 (81.0) | 0 (0.0) | 0 (0.0) | |
| 66–80 | 115 (61.2) | 17 (5.1) | 0 (0.0) | 241 (75.1) | |
| > 80 | 62 (33.0) | 0 (0.0) | 0 (0.0) | 80 (24.9) | |
| Department | |||||
| Neuro | 92 (48.9) | 145 (43.8) | 61 (56.5) | 136 (42.4) | 0.001 |
| Surgical | 72 (38.3) | 152 (45.9) | 40 (37.0) | 164 (51.1) | |
| Medical | 21 (11.2) | 22 (6.6) | 6 (5.6) | 8 (2.5) | |
| Other | 3 (1.6) | 12 (3.6) | 1 (0.9) | 13 (4.0) | |
| Hospital admission | |||||
| From home | 110 (58.5) | 216 (65.3) | 76 (70.4) | 211 (65.7) | 0.001 |
| From hospital | 65 (34.6) | 113 (34.1) | 31 (28.7) | 104 (32.4) | |
| From nursing home | 12 (6.4) | 2 (0.6) | 0 | 4 (1.2) | |
| Unknown | 1 (0.5) | 0 | 1 (0.9) | 2 (0.6) | |
| Scorings | |||||
| Frailty | 101 (53.7) | 44 (13.3) | 13 (12.0) | 88 (27.4) | < 0.001 |
| Glasgow Coma Scale | 6 [3–10] | 14 [7–15] | 14 [7–15] | 15 [14, 15] | < 0.001 |
| APACHE 2 | 24 [20–28] | 14 [9–17] | 9 [4–14] | 13 [10–15] | < 0.001 |
| SOFA | 10 [7–12] | 6 [4–8] | 5 [3–8] | 6 [4–7] | < 0.001 |
| Charlson Comorbidity Index | 2 [1–4] | 1 [0–2] | 0 [0–1] | 2 [1–3] | < 0.001 |
| ICU admission reasons | |||||
| Sepsis | 48 (25.5) | 39 (11.8) | 11 (10.2) | 39 (12.1) | < 0.001 |
| Polytrauma | 2 (1.1) | 8 (2.4) | 18 (16.7) | 7 (2.2) | < 0.001 |
| Traumatic brain injury | 22 (11.7) | 28 (8.5) | 25 (23.1) | 34 (10.6) | < 0.001 |
| Non-traumatic brain injury | 46 (24.5) | 90 (27.2) | 28 (25.9) | 52 (16.2) | 0.006 |
| Postoperative care | 19 (10.1) | 68 (20.5) | 19 (17.6) | 84 (26.2) | < 0.001 |
| Cardiac failure | 18 (9.6) | 16 (4.8) | 2 (1.9) | 18 (5.6) | 0.034 |
| Respiratory failure | 62 (33.0) | 90 (27.2) | 20 (18.5) | 117 (36.4) | 0.002 |
| Other admission reasons | 18 (9.6) | 63 (19.0) | 9 (8.3) | 63 (19.6) | 0.001 |
| MTB at hospital admission | 30 [15–30] | 30 [30–30] | 30 [30–30] | 30 [30–30] | < 0.001 |
| 0 | 3 (1.6) | 4 (1.2) | 2 (1.9) | 0 (0.0) | |
| 5 | 13 (6.9) | 2 (0.6) | 2 (1.9) | 2 (0.6) | |
| 10 | 11 (5.9) | 2 (0.6) | 5 (4.6) | 3 (0.9) | |
| 15 | 21 (11.2) | 6 (1.8) | 1 (0.9) | 9 (2.8) | < 0.001 |
| 20 | 24 (12.8) | 8 (2.4) | 1 (0.9) | 12 (3.7) | |
| 25 | 19 (10.1) | 11 (3.3) | 1 (0.9) | 29 (9.0) | |
| 30 | 97 (51.6) | 298 (90.0) | 96 (88.9) | 266 (82.9) | |
Data is presented as n (%) or median (interquartile range). Frailty is assumed at Clinical Frailty Scale ≥ 5. A p-value of < 0.05 was considered significant
APACHE Acute physiology and chronic health evaluation score, SOFA Sepsis-related organ failure assessment score, MTB Mobility-Transfer-Barthel [32]
Mobilization parameters of the different cluster
Each parameter used to characterize mobilization differed significantly between the clusters (Table 2). Early mobilization (within the first 72 h after ICU admission) was applied to all clusters (Table 2). The dosage of mobilization differed significantly between the clusters. Patients of the cluster “Old non-frail” accomplished the longest mean daily duration (28 min [9–67 min]), those of the cluster “Young Trauma” achieved the highest maximum SOMS level (3 [2–4]), those of cluster “Severely ill & Frail” the lowest maximum SOMS level (2 [1–3]) and had the shortest mean duration of their mobilization session per day (6 min [2–27 min]. Univariate and post hoc analyses are presented in Additional file 1: Table S3.
Table 2.
Mobilization parameters in the four clusters
| Severely ill & Frail | Middle-aged | Young Trauma | Old non-frail | P value | |
|---|---|---|---|---|---|
| (N = 188) | (N = 331) | (N = 108) | (N = 321) | ||
| Frequency of mobilization session per day | 1.1 (1.0–1.2) | 1.2 (1.0–1.4) | 1.2 (1.0–1.5) | 1.2 (1.0–1.4) | < 0.001 |
| Mean duration per day (min) | 6 (2–27) | 13 (5–39) | 20 (7–51) | 28 (9–67) | < 0.001 |
| First day of mobilization | 4 (3–7) | 3 (2–7) | 3 (2–7) | 2 (2–4) | < 0.001 |
| Early mobilization | 79 (42.0) | 187 (56.5) | 62 (57.4) | 233 (72.6) | < 0.001 |
| Maximum SOMS level | 2 (1–3) | 3 (2, 3) | 3 (2–4) | 3 (2–3) | < 0.001 |
| 0 | 7 (3.7) | 9 (2.7) | 0 | 4 (1.2) | < 0.001 |
| 1 | 67 (35.6) | 67 (20.2) | 10 (9.3) | 35 (10.9) | |
| 2 | 61 (32.4) | 82 (24.8) | 26 (24.1) | 91 (28.3) | |
| 3 | 34 (18.1) | 89 (26.9) | 35 (32.4) | 126 (39.3) | |
| 4 | 19 (10.1) | 84 (25.4) | 37 (34.3) | 65 (20.2) |
Data is presented as n (%) or median (interquartile range). Early mobilization is given, if the first session was performed earlier than 72h after ICU admission. A p-value of < 0.05 was considered significant. SOMS Surgical Intensive Care Unit Optimal Mobilization Score (ranging from 0 to 4) [18–20]
Primary outcome
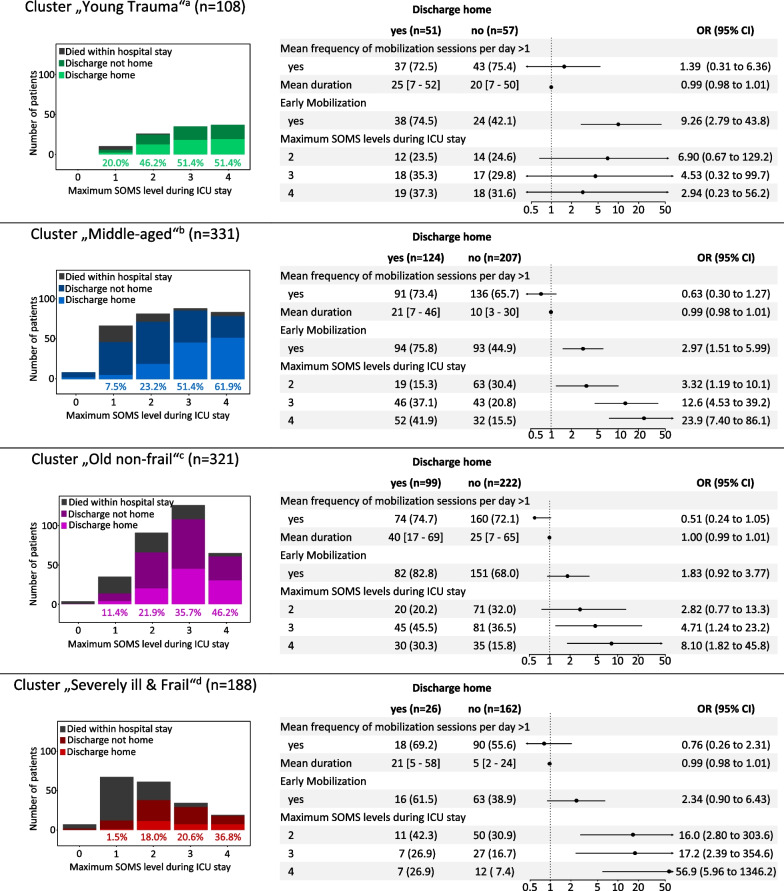
The influence of mobilization on the discharge disposition home differed significantly between the clusters. Early mobilization (< 72 h) was the most significant factor in the cluster “Young Trauma” (ORadj 10.0 [2.8 to 44.0], p < 0.001) and in the cluster of “Middle-aged” patients (cluster 2, ORadj 3.0 (95%CI [1.5 to 6.0]), p < 0.001), whereas there was no significant influence in the other clusters (Fig. 3). In the clusters “Middle-aged” and “Severely ill & Frail,” the likelihood of being discharged home increased with each SOMS level achieved, up to a 24-fold increased likelihood with SOMS level 4 (ORadj 23.9 [7.4 to 86.1], p < 0.001 and ORadj 56.9 [6.9 to 1346.2], p = 0.002, respectively). In the cluster “Old non-frail,” the probability to be discharged home increased significantly, if SOMS > 3 was achieved (ORadj 4.7 [1.2 to 23.2], p = 0.035 and ORadj 8.1 [1.8 to 45.8], p = 0.010, respectively) (Fig. 3). The logistic regression models for possible confounders are given in Additional file 1: Table S4, the sensitivity analysis with survivors only in Additional file 1: Table S5 and the 11-point IMS results that confirm the results of the primary analysis in Additional file 1: Table S6.
Fig. 3.
Synthetic figure summarizing the main findings. The bar charts show the number of patients in each maximum achieved SOMS level according to their discharge disposition. The percentages below the columns show the frequency of patients discharged home of each SOMS level. Numbers are presented as n (%) or median [IQR]. Early mobilization is defined as mobilization within the first 72 h after ICU admission. The reference for early mobilization is “No Early Mobilization,” the reference for maximum SOMS level achieved is “0/1″. aModel was corrected for “Hospital admission,” “Body Mass Index (categories),” “Clinical Frailty Scale,” “Other ICU admission reasons,” “Postoperative care” and “SOFA.” bModel was corrected for “Hospital admission,” “APACHE,” “Body Mass Index (categories),” “Charlson Comorbidity Index,” “Clinical Frailty Scale,” “Other ICU admission reasons” and “SOFA.” cModel was corrected for “Hospital admission,” “Age (categories),” “APACHE,” “Mobility-Transfer-Barthel” and “Department.” dModel was corrected for “Clinical Frailty Scale” and “Postoperative care.” ICU Intensive care unit, IQR Interquartile range, SOMS-Score Surgical Intensive Care Unit Optimal Mobilization Score [18–20]
Secondary outcomes
The probability to be discharged home as well as hospital mortality differed significantly between the clusters in the univariate analysis (Fig. 3 and Additional file 1: Table S7). ICU and hospital mortality were the highest in the cluster “Severely ill & Frail” (34% and 42%, p < 0.001, respectively), whereas in the cluster “Young Trauma” mortality was only 1.1% (for details Additional file 1: Table S7).
Discussion
In this study, critically ill patients were homogeneously clustered with their clinical and procedural characteristics to evaluate the personalized benefits of the components of mobilization therapy for discharge home using a machine learning technology. The resulting four clusters (“Severely ill & Frail,” “Middle-aged,” “Young Trauma” and “Old non-frail”) differed in the components of mobilization and in the frequency of discharge home. In the clusters “Young Trauma” and “Middle-aged,” early mobilization was strongly associated with discharge home. In the clusters “Middle-aged,” “Old non-frail” and “Severely ill & Frail,” the achieved level of mobilization during ICU stay indicated the best chance of discharge home.
While studies have shown that early mobilization improves short-term patient outcomes [3–5, 36], the individual optimal dose of mobilization is unknown. Using the FITT principle, the mobilization dose can be specified by frequency, intensity, time and modality [10, 37]. Whereas reporting of these components was mostly incomplete across studies, in our prospectively designed observational database, they were explicitly queried.
Since there is still no uniform definition when to start early mobilization [38], we used definition of the German guideline, i.e., start within 72 h of ICU admission, which was also confirmed beneficial in a network-metanalysis [25, 39]. While early mobilization was performed in all four clusters, a significant effect on outcome was only evident in the clusters of “Young Trauma” and “Middle-aged” patients.
There is no evidence on the optimal frequency of mobilization. In their prospective, randomized clinical trial, Winkelmann et al. found no benefit of two mobilization sessions compared to one [40]. In accordance, Paton et al. also found that health six months after ICU therapy was not better when patients were treated with more mobilization sessions per day [15]. Also, Scheffenbichler et al. concluded in their investigation of surgical critically ill patients that level and duration were modifying outcome but not frequency [9]. In our study, however, patients of the cluster “Old non-frail” benefited from more frequent mobilization sessions. As is known from stroke patients, elderly patients may benefit from more frequent but shorter sessions thus avoiding overuse given their lower physiological reserves [41].
Most important for the patients seemed to be the achieved level of mobilization in the ICU. Except for the “Young Trauma” cluster, all other clusters benefited significantly from higher levels of mobilization. Especially in the cluster “Severely ill & Frail,” patients achieving SOMS levels ≥ 2 (sitting) had lower mortality and a higher chance of being discharged home. These findings are aligned with all other studies investigating level. Paton et al. [15] and also Scheffenbichler et al. [9] found that the ability to stand (SOMS ≥ 3) in the ICU was an important milestone for improving 6-month outcomes. Dos Santos Moraes et al. showed that patients, who achieved high IMS scores, had significantly increased likelihood of being discharged home and a reduced probability of in-hospital death. Unfortunately, the authors did not include data of prehospital functional status or comorbidities in their analysis [14].
This shortcoming was addressed by Mayer et al., who demonstrated that ECMO patients achieving higher mobility levels had a better chance to survive [42]. However, the authors raised the question of whether early mobilization actually improves outcome and whether the more favorable disease course does not allow mobilization with more rapid progression to higher levels [42]. Therefore, we adjusted mobilization parameters for disease severity, age and other influencing factors. Importantly, mobilization results were not altered by these adjustments, underscoring the relevance of this intervention.
Most interestingly duration did not modify the effect on any of our clusters which is also important for resource allocation. In contrast, Scheffenbichler et al. demonstrated that duration had a positive impact [9]. The authors pointed out that their findings contrast with those of stroke patients who were treated in stroke units but did not require intensive care. Here, the length of mobilization in particular had a negative effect on outcome if the sessions were not split up in several short sessions [41]. Although our unsupervised learning approach did not identify a cluster of exclusively neurocritical patients, they were included in this study. Nevertheless, duration of mobilization should be investigated in future studies.
In summary, for each of the four clusters, different effect modifications of mobilization components on discharge disposition could be demonstrated. This emphasizes the relevance of subdividing the heterogeneous cohort of critically ill patients, e.g., by functionality and pre-existing frailty or independence.
We recognize some key limitations. Generalizability is limited as a single-center study. However, the aim of this study was feasibility of an individual approach to mobilization. External validation will be a future aim. Of particular importance here is the extent, to which a causality between mobilization and outcome can be confirmed or whether the outcome is driven by patient characteristics. However, by adjusting the models for disease severity, age and previous health conditions, the clusters are homogeneous with respect to these factors. Although an even finer differentiation might provide more individualization, it would have resulted in very small clusters that are difficult to define clinically. Third, a differentiation of the modality of mobilization as described in the FITT principle was not part of our analysis. However, mobilization was recorded independent of the executing staff (e.g., physiotherapy, nurses, doctors) with details of timing, level and duration. In addition, barriers to mobilization or discontinuation criteria were not recorded in this study.
Conclusion
Using machine learning, an identification of defined patient clusters was possible. These four clusters (“Severely ill & Frail,” “Middle-aged,” “Young Trauma” and “Old non-frail”) had different clinical characteristics. Furthermore, different mobilization components were important for the respective cluster's outcome. After external and prospective evaluation, this clustering may allow to individualize mobilization of critically ill patients and improve outcome. Competing risk analyses including time-dependent variables may further help to understand how the trajectory of intensive care and the course of mobilization interact with respect to outcome.
Supplementary Information
Additional file 1. Figure S1: Elbow method for determination of clusters. Figure S2: Euclidian distance plot. Heatmap showing Euclidean distances between samples clustered using complete linkage. Factors used for Cluster Analysis are visualized as column annotations. Table S1: Patient Characteristics. Numbers are presented as n (%) or median [IQR]. “Frail” is defined as Clinical Frailty Scale 5–9. A p-value of < 0.05 was considered significant. ICU Intensive Care Unit, IQR Interquartile Range, APACHE II Acute Physiology and Chronic Health Evaluation Score, SOFA Sepsis-related Organ Failure Assessment Score, MTB Mobility-Transfer-Barthel. Table S2: Post-hoc Analyses of patient characteristics in the four clusters. Numbers are presented as n (%) or median [IQR]. “Frail” is defined as Clinical Frailty Scale 5–9. A p-value of < 0.05 was considered significant. APACHE Acute Physiology and Chronic Health Evaluation Score, SOFA Sepsis-related Organ Failure Assessment Score, MTB Mobility-Transfer-Barthel. Table S3: Post-hoc Analyses of mobilization characteristics in the four clusters. Early mobilization is defined as mobilization within 72h of ICU admission. A p-value of < 0.05 was considered significant. Numbers are presented as n (%) or median [IQR]. IQR Interquartile Range, SOMS Surgical Intensive Care Unit Optimal Mobilization Score. Table S4: Stepwise Regression Models. “Frailty” is defined as Clinical Frailty Scale 5–9. A p-value of < 0.05 was considered significant. Early mobilization is defined as mobilization within 72h of ICU admission. OR Odds Ratio, CI Confidence interval, ICU Intensive Care Unit, IQR Interquartile Range, APACHE II Acute Physiology and Chronic Health Evaluation Score, SOFA Sepsisrelated Organ Failure Assessment Score, SOMS Surgical Intensive Care Unit Optimal Mobilization Score. Table S5: Logistic regression models of survivors of hospital stay. Early mobilization is defined as mobilization within 72h of ICU admission. A p-value of < 0.05 was considered significant. OR Odds Ratio, CI Confidence interval, SOMS Surgical Intensive Care Unit Optimal Mobilization Score. a Model was corrected for postoperative care, frailty, department. b Model was corrected for Sepsis-related Organ Failure Assessment Score, body mass index (categories), postoperative, frailty, other admission reasons, admission, and non-traumatic brain injury. c Model was corrected for department and Glasgow Coma Scale. d Model was corrected for APACHE II, department, admission, Mobility-Transfer-Barthel Score at hospital admission and age (categories). Table S6: Sensitivity Analysis with the ICU Mobility Scale. Early mobilization is defined as mobilization within 72h of ICU admission. A p-value of < 0.05 was considered significant. OR Odds Ratio, CI Confidence interval. IQR Interquartile Range, IMS ICU Mobility Scale. Table S7: Secondary Endpoints. Secondary endpoints in dependence of the four clusters. Here, the results of the primary analysis as well as the post-hoc analyses are listed. Numbers are presented as n (%) or median [IQR]. A p-value of < 0.05 was considered significant. IQR Interquartile Range, ICU Intensive Care Unit.
Author contributions
SJS is the principal investigator and developed the protocol, BU is the study statistician. SJS and MB were involved in the ethical approval, KEF, BU, MB and SJS were involved in the analysis and interpretation of the data. KEF, SG, KB and SJS were involved in data acquisition and quality assurance. All authors critically revised the manuscript and approved its final version.
Funding
Open Access funding enabled and organized by Projekt DEAL. Institutional funds only.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of the School of Medicine of the Technical University of Munich (Grillparzer Str. 16, 81675 Munich, Chairperson Prof. Dr. G. Schmidt, approval number 518/16S). The study was conducted in agreement with the principles of the Declaration of Helsinki. All participants were informed about the purpose of the study, the risks, and the potential benefits. Written informed consent was obtained by the local study physician from each participant for the original studies. The Department of Anaesthesiology and Intensive Care of the School of Medicine, Technical University of Munich coordinated the study.
Consent for publication
Not applicable.
Competing interests
MB received research support from MSD (Haar, Germany) not related to this manuscript, received honoraria for giving lectures from GE Healthcare (Helsinki, Finland) and Grünenthal (Aachen, Germany). SJS received grants and non-financial support from Reactive Robotics GmbH (Munich, Germany), ASP GmbH (Attendorn, Germany), STIMIT AG (Biel, Switzerland), ESICM (Geneva, Switzerland), grants, personal fees and non-financial support from Fresenius Kabi Deutschland GmbH (Bad Homburg, Germany), personal fees from Springer Verlag GmbH (Vienna, Austria) for educational purposes and Advanz Pharma GmbH (Bielefeld, Germany), non-financial support from national and international societies (and their congress organizers) in the field of anesthesiology and intensive care medicine, outside the submitted work. Dr. Schaller held stocks in small amounts from Rhön-Klinikum AG and holds stocks in small amounts from Alphabeth Inc., Bayer AG and Siemens AG; these holdings have not affected any decisions regarding his research or this study. The other authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang L, Hu W, Cai Z, Liu J, Wu J, Deng Y, Yu K, Chen X, Zhu L, Ma J, et al. Early mobilization of critically ill patients in the intensive care unit: a systematic review and meta-analysis. PLoS ONE. 2019;14(10):e0223185. doi: 10.1371/journal.pone.0223185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nydahl P, Sricharoenchai T, Chandra S, Kundt FS, Huang M, Fischill M, Needham DM. Safety of patient mobilization and rehabilitation in the intensive care unit systematic review with meta-analysis. Ann Am Thorac Soc. 2017;14(5):766–777. doi: 10.1513/AnnalsATS.201611-843SR. [DOI] [PubMed] [Google Scholar]
- 3.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaller SJ, Anstey M, Blobner M, Edrich T, Grabitz SD, Gradwohl-Matis I, Heim M, Houle T, Kurth T, Latronico N, et al. Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet. 2016;388(10052):1377–1388. doi: 10.1016/S0140-6736(16)31637-3. [DOI] [PubMed] [Google Scholar]
- 5.Klem HE, Tveiten TS, Beitland S, Malerod S, Kristoffersen DT, Dalsnes T, Nupen-Stieng MB, Larun L. Early activity in mechanically ventilated patients - a meta-analysis. Tidsskr Nor Laegeforen. 2021 doi: 10.4045/tidsskr.20.0351. [DOI] [PubMed] [Google Scholar]
- 6.Hodgson CL, Bailey M, Bellomo R, Brickell K, Broadley T, Buhr H, Gabbe BJ, Gould DW, Harrold M, Higgins AM, et al. Early active mobilization during mechanical ventilation in the ICU. N Engl J Med. 2022;387(19):1747–1758. doi: 10.1056/NEJMoa2209083. [DOI] [PubMed] [Google Scholar]
- 7.Moss M. Early mobilization of critical care patients - still more to learn. N Engl J Med. 2022;387(19):1807–1808. doi: 10.1056/NEJMe2212360. [DOI] [PubMed] [Google Scholar]
- 8.Parry SM, Remedios L, Denehy L, Knight LD, Beach L, Rollinson TC, Berney S, Puthucheary ZA, Morris P, Granger CL. What factors affect implementation of early rehabilitation into intensive care unit practice? A qualitative study with clinicians. J Crit Care. 2017;38:137–143. doi: 10.1016/j.jcrc.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Scheffenbichler FT, Teja B, Wongtangman K, Mazwi N, Waak K, Schaller SJ, Xu X, Barbieri S, Fagoni N, Cassavaugh J, et al. Effects of the level and duration of mobilization therapy in the surgical ICU on the loss of the ability to live independently: an international prospective cohort study. Crit Care Med. 2021;49(3):e247–e257. doi: 10.1097/CCM.0000000000004808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ammann BC, Knols RH, Baschung P, de Bie RA, de Bruin ED. Application of principles of exercise training in sub-acute and chronic stroke survivors: a systematic review. BMC Neurol. 2014;14:167. doi: 10.1186/s12883-014-0167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brock C, Marzano V, Green M, Wang J, Neeman T, Mitchell I, Bissett B. Defining new barriers to mobilisation in a highly active intensive care unit - have we found the ceiling? An Observ Stud Heart Lung. 2018;47(4):380–385. doi: 10.1016/j.hrtlng.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Pohlman MC, Schweickert WD, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D, et al. Feasibility of physical and occupational therapy beginning from initiation of mechanical ventilation. Crit Care Med. 2010;38(11):2089–2094. doi: 10.1097/CCM.0b013e3181f270c3. [DOI] [PubMed] [Google Scholar]
- 13.Scheffenbichler L, Teja B, Scheffenbichler F, Blobner M, Houle T, Eikermann M. SOMS team: influence of the acuity of patients' illness on effectiveness of early, goal-directed mobilization in the intensive care unit: a post hoc analysis. Crit Care. 2020;24(1):663. doi: 10.1186/s13054-020-03346-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dos Santos Moraes TL, de Farias JMF, Rezende BS, de Carvalho FO, Santiago MS, Porto ES, Doria FM, Santana KCS, Gomes MV, Leite VS, et al. Limited mobility to the bed reduces the chances of discharge and increases the chances of death in the ICU. Clin Pract. 2021;12(1):8–16. doi: 10.3390/clinpract12010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paton M, Lane R, Paul E, Cuthburtson GA, Hodgson CL. Mobilization during critical illness: a higher level of mobilization improves health status at 6 months, a secondary analysis of a prospective cohort study. Crit Care Med. 2021;49(9):e860–e869. doi: 10.1097/CCM.0000000000005058. [DOI] [PubMed] [Google Scholar]
- 16.Conti M, Friolet R, Eckert P, Merlani P. Home return 6 months after an intensive care unit admission for elderly patients. Acta Anaesthesiol Scand. 2011;55(4):387–393. doi: 10.1111/j.1399-6576.2011.02397.x. [DOI] [PubMed] [Google Scholar]
- 17.Hoyer EH, Needham DM, Atanelov L, Knox B, Friedman M, Brotman DJ. Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. 2014;9(5):277–282. doi: 10.1002/jhm.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaller SJ, Stauble CG, Suemasa M, Heim M, Duarte IM, Mensch O, Bogdanski R, Lewald H, Eikermann M, Blobner M. The German validation study of the surgical intensive care unit optimal mobility score. J Crit Care. 2016;32:201–206. doi: 10.1016/j.jcrc.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 19.Schaller SJ, Scheffenbichler FT, Bose S, Mazwi N, Deng H, Krebs F, Seifert CL, Kasotakis G, Grabitz SD, Latronico N, et al. Influence of the initial level of consciousness on early, goal-directed mobilization: a post hoc analysis. Intensive Care Med. 2019;45(2):201–210. doi: 10.1007/s00134-019-05528-x. [DOI] [PubMed] [Google Scholar]
- 20.Kasotakis G, Schmidt U, Perry D, Grosse-Sundrup M, Benjamin J, Ryan C, Tully S, Hirschberg R, Waak K, Velmahos G, et al. The surgical intensive care unit optimal mobility score predicts mortality and length of stay. Crit Care Med. 2012;40(4):1122–1128. doi: 10.1097/CCM.0b013e3182376e6d. [DOI] [PubMed] [Google Scholar]
- 21.Hodgson C, Needham D, Haines K, Bailey M, Ward A, Harrold M, Young P, Zanni J, Buhr H, Higgins A, et al. Feasibility and inter-rater reliability of the ICU mobility scale. Heart Lung. 2014;43(1):19–24. doi: 10.1016/j.hrtlng.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Hodgson C, Bailey M, Bellomo R, Berney S, Buhr H, Denehy L, Gabbe B, Harrold M, Higgins A, Iwashyna T, et al. A binational multicenter pilot feasibility randomized controlled trial of early goal-directed mobilization in the ICU. Critic Care Med. 2016;44(6):1145–1152. doi: 10.1097/CCM.0000000000001643. [DOI] [PubMed] [Google Scholar]
- 23.Tipping CJ, Bailey MJ, Bellomo R, Berney S, Buhr H, Denehy L, Harrold M, Holland A, Higgins AM, Iwashyna TJ, et al. The ICU mobility scale has construct and predictive validity and is responsive a multicenter observational study. Ann Am Thorac Soc. 2016;13(6):887–893. doi: 10.1513/AnnalsATS.201510-717OC. [DOI] [PubMed] [Google Scholar]
- 24.Fuest K, Schaller SJ. Early mobilisation on the intensive care unit : what we know. Med Klin Intensivmed Notfmed. 2019;114(8):759–764. doi: 10.1007/s00063-019-0605-4. [DOI] [PubMed] [Google Scholar]
- 25.Bein T, Bischoff M, Bruckner U, Gebhardt K, Henzler D, Hermes C, Lewandowski K, Max M, Nothacker M, Staudinger T, et al. S2e guideline: positioning and early mobilisation in prophylaxis or therapy of pulmonary disorders : revision 2015: S2e guideline of the German society of anaesthesiology and intensive care medicine (DGAI) Anaesthesist. 2015;64(Suppl 1):1–26. doi: 10.1007/s00101-015-0071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flaatten H, De Lange DW, Morandi A, Andersen FH, Artigas A, Bertolini G, Boumendil A, Cecconi M, Christensen S, Faraldi L, et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (>/= 80 years) Intensive Care Med. 2017;43(12):1820–1828. doi: 10.1007/s00134-017-4940-8. [DOI] [PubMed] [Google Scholar]
- 27.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57(12):1288–1294. doi: 10.1016/j.jclinepi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intens Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 30.Mahoney FI, Barthel DW. Functional evaluation: the barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 31.Lubke N, Meinck M, Von Renteln-Kruse W. The barthel index in geriatrics a context analysis for the Hamburg classification manual. Z Gerontol Geriatr. 2004;37(4):316–326. doi: 10.1007/s00391-004-0233-2. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe S, Kotani T, Taito S, Ota K, Ishii K, Ono M, Katsukawa H, Kozu R, Morita Y, Arakawa R, et al. Determinants of gait independence after mechanical ventilation in the intensive care unit: a Japanese multicenter retrospective exploratory cohort study. J Intensive Care. 2019;7:53. doi: 10.1186/s40560-019-0404-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sammouda R, El-Zaart A. An optimized approach for prostate image segmentation using K-means clustering algorithm with elbow method. Comput Intell Neurosci. 2021;2021:4553832. doi: 10.1155/2021/4553832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hennig C. Dissolution point and isolation robustness: robustness criteria for general cluster analysis methods. J Multivar Anal. 2008;99(6):1154–1176. doi: 10.1016/j.jmva.2007.07.002. [DOI] [Google Scholar]
- 35.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and cox regression. Am J Epidemiol. 2007;165(6):710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 36.Waldauf P, Jiroutkova K, Krajcova A, Puthucheary Z, Duska F. Effects of rehabilitation interventions on clinical outcomes in critically Ill patients: systematic review and meta-analysis of randomized controlled trials. Crit Care Med. 2020;48(7):1055–1065. doi: 10.1097/CCM.0000000000004382. [DOI] [PubMed] [Google Scholar]
- 37.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP. American college of sports medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 38.Clarissa C, Salisbury L, Rodgers S, Kean S. Early mobilisation in mechanically ventilated patients: a systematic integrative review of definitions and activities. J Intensive Care. 2019;7:3. doi: 10.1186/s40560-018-0355-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding N, Zhang Z, Zhang C, Yao L, Yang L, Jiang B, Wu Y, Jiang L, Tian J. What is the optimum time for initiation of early mobilization in mechanically ventilated patients? A network meta-analysis. PLoS ONE. 2019;14(10):e0223151. doi: 10.1371/journal.pone.0223151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winkelman C, Sattar A, Momotaz H, Johnson KD, Morris P, Rowbottom JR, Thornton JD, Feeney S, Levine A. Dose of early therapeutic mobility: does frequency or intensity matter? Biol Res Nurs. 2018;20(5):522–530. doi: 10.1177/1099800418780492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernhardt J, Churilov L, Ellery F, Collier J, Chamberlain J, Langhorne P, Lindley RI, Moodie M, Dewey H, Thrift AG, et al. Prespecified dose-response analysis for a very early rehabilitation Trial (AVERT) Neurology. 2016;86(23):2138–2145. doi: 10.1212/WNL.0000000000002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayer KP, Pastva AM, Du G, Hatchett SP, Chang M, Henning AN, Maher B, Morris PE, Zwischenberger JB. Mobility levels with physical rehabilitation delivered during and after extracorporeal membrane oxygenation: a marker of illness severity or an indication of recovery? Phys Ther. 2022;102(3):301. doi: 10.1093/ptj/pzab301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Figure S1: Elbow method for determination of clusters. Figure S2: Euclidian distance plot. Heatmap showing Euclidean distances between samples clustered using complete linkage. Factors used for Cluster Analysis are visualized as column annotations. Table S1: Patient Characteristics. Numbers are presented as n (%) or median [IQR]. “Frail” is defined as Clinical Frailty Scale 5–9. A p-value of < 0.05 was considered significant. ICU Intensive Care Unit, IQR Interquartile Range, APACHE II Acute Physiology and Chronic Health Evaluation Score, SOFA Sepsis-related Organ Failure Assessment Score, MTB Mobility-Transfer-Barthel. Table S2: Post-hoc Analyses of patient characteristics in the four clusters. Numbers are presented as n (%) or median [IQR]. “Frail” is defined as Clinical Frailty Scale 5–9. A p-value of < 0.05 was considered significant. APACHE Acute Physiology and Chronic Health Evaluation Score, SOFA Sepsis-related Organ Failure Assessment Score, MTB Mobility-Transfer-Barthel. Table S3: Post-hoc Analyses of mobilization characteristics in the four clusters. Early mobilization is defined as mobilization within 72h of ICU admission. A p-value of < 0.05 was considered significant. Numbers are presented as n (%) or median [IQR]. IQR Interquartile Range, SOMS Surgical Intensive Care Unit Optimal Mobilization Score. Table S4: Stepwise Regression Models. “Frailty” is defined as Clinical Frailty Scale 5–9. A p-value of < 0.05 was considered significant. Early mobilization is defined as mobilization within 72h of ICU admission. OR Odds Ratio, CI Confidence interval, ICU Intensive Care Unit, IQR Interquartile Range, APACHE II Acute Physiology and Chronic Health Evaluation Score, SOFA Sepsisrelated Organ Failure Assessment Score, SOMS Surgical Intensive Care Unit Optimal Mobilization Score. Table S5: Logistic regression models of survivors of hospital stay. Early mobilization is defined as mobilization within 72h of ICU admission. A p-value of < 0.05 was considered significant. OR Odds Ratio, CI Confidence interval, SOMS Surgical Intensive Care Unit Optimal Mobilization Score. a Model was corrected for postoperative care, frailty, department. b Model was corrected for Sepsis-related Organ Failure Assessment Score, body mass index (categories), postoperative, frailty, other admission reasons, admission, and non-traumatic brain injury. c Model was corrected for department and Glasgow Coma Scale. d Model was corrected for APACHE II, department, admission, Mobility-Transfer-Barthel Score at hospital admission and age (categories). Table S6: Sensitivity Analysis with the ICU Mobility Scale. Early mobilization is defined as mobilization within 72h of ICU admission. A p-value of < 0.05 was considered significant. OR Odds Ratio, CI Confidence interval. IQR Interquartile Range, IMS ICU Mobility Scale. Table S7: Secondary Endpoints. Secondary endpoints in dependence of the four clusters. Here, the results of the primary analysis as well as the post-hoc analyses are listed. Numbers are presented as n (%) or median [IQR]. A p-value of < 0.05 was considered significant. IQR Interquartile Range, ICU Intensive Care Unit.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.