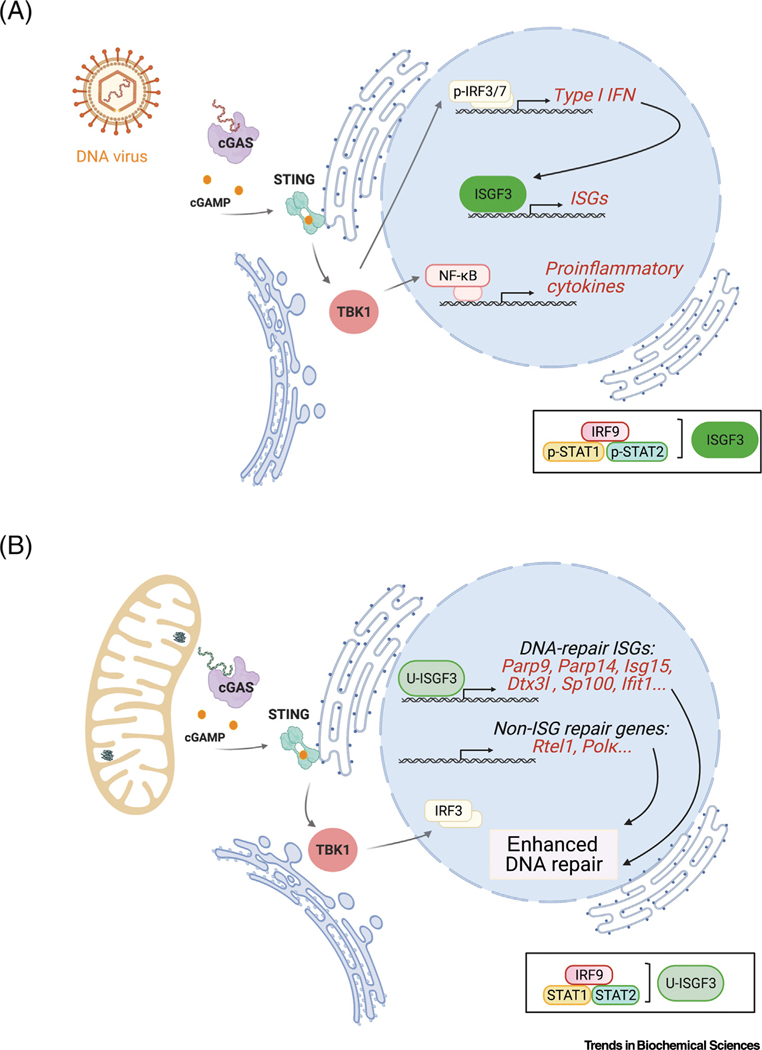
Figure 1. DNA-mediated innate immune and mtDNA-mediated genotoxic stress signaling.
(A) Upon DNA virus infection, the cellular DNA sensor cyclic GMP-AMP synthase (cGAS) (purple) binds the viral DNA and produces the small cyclic dinucleotide, 2′−3′-cyclic GMP-AMP (cGAMP) (small circle). Stimulator of interferon genes (STING) [shown at the endoplasmic reticulum (ER)] binds cGAMP and subsequently activates TBK1 (at the ER and Golgi intermediate compartment), resulting in the nuclear localization of IRF3, IRF7, and NF- B to activate transcription of type I interferon (IFN) and proinflammatory cytokines. Activation of type I IFN signaling leads to the formation of the ISGF3 transcription factor complex (green), composed of phosphorylated STAT1 (p-STAT1), phosphorylated STAT2 (p-STAT2), and IRF9 (see box below blue nucleus). ISGF3 induces expression of many IFN-stimulated genes (ISGs) as part of the antiviral response.
(B) Similar to viral DNA, mtDNA released into the cytoplasm binds cGAS and activates STING and TBK1. However, in Tfam+/– cells, where this pathway was unveiled, this instead leads to IRF3-dependent formation of the U-ISGF3 complex (composed of unphosphorylated STAT1, STAT2, and IRF9, see box below blue nucleus), which drives expression of a unique subset of ISGs (e.g., Parp9, Parp14, Isg15, Dtx3l, sp100, and Ifit1) that enhance nDNA repair. Other DNA repair-associated genes (e.g., Rtel1 and Pol ) are also upregulated in Tfam+/– cells, that we propose, along with the DNA repair-associated ISGs, form an ‘mtDNA damage response regulon’ that enhances nDNA repair as part of the genotoxic stress sentinel function of mtDNA.