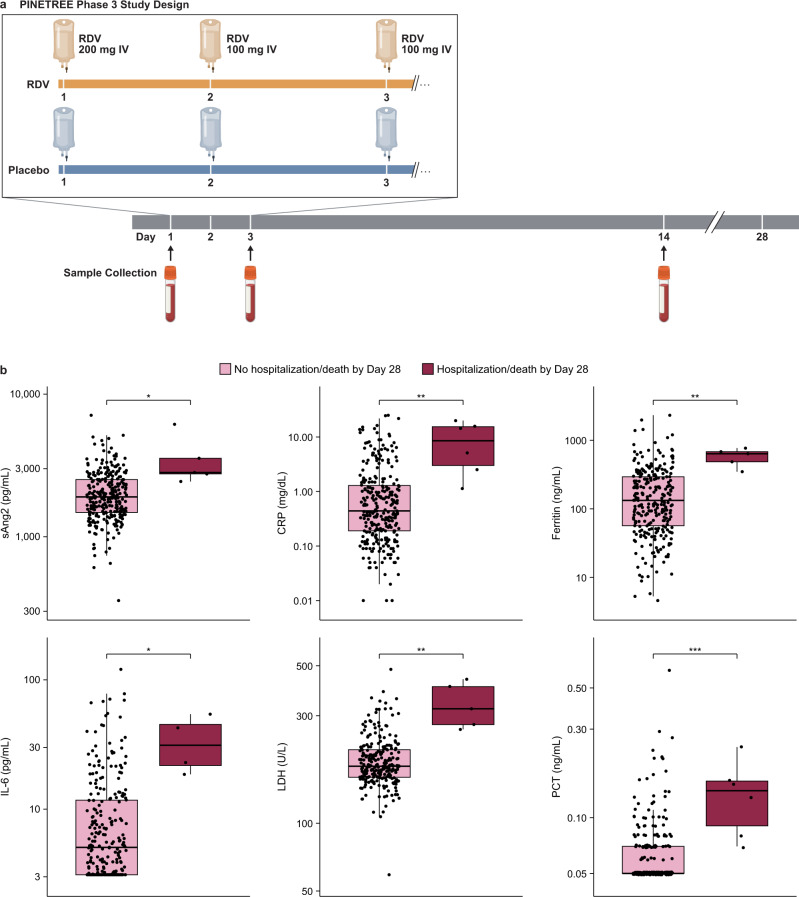
Fig. 1. Baseline inflammation biomarkers associated with the primary endpoint of the PINETREE study.
a Design of the PINETREE study, a randomized, double-blind, placebo-controlled Phase 3 clinical trial in patients at high-risk of severe COVID-19. Participants were randomly assigned (1:1) to receive remdesivir (RDV) or placebo every day for 3 doses. Serum and plasma were collected for biomarker evaluation in a subset of participants at day 1, day 3, and day 14. b Box plots of significantly different (FDR < 0.05) biomarkers at baseline (day 1) in patients who met the primary endpoint of hospitalization or death by day 28 (purple, soluble Angiopoietin 2 (sAng2): n = 288; ferritin: n = 275; interleukin-6 (IL-6): n = 267; lactate dehydrogenase (LDH): n = 246; C-reactive protein (CRP): n = 289; procalcitonin (PCT): n = 288) vs. those who did not (pink; IL-6: n = 4; sAng2, ferritin, LDH: n = 5; CRP, PCT: n = 6). *FDR < 0.05; **FDR < 0.01; ***FDR < 0.001, ****FDR < 0.0001. The line within each box denotes the median and each box extends to the 25th and 75th percentiles. The whiskers indicate 1.5 interquartile range. Significance was determined using a Wilcoxon Rank Sum Test.