Figure 6.
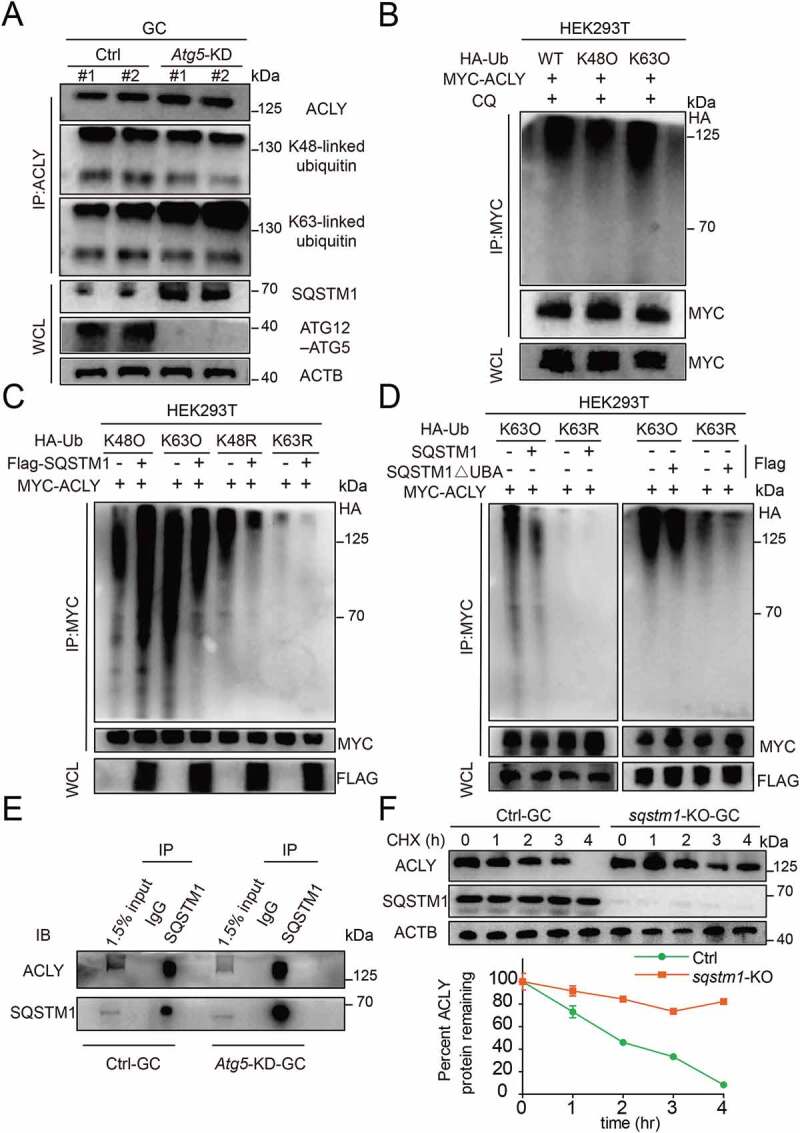
K63-linked polyubiquitination of ACLY facilitates its selective autophagic degradation by interacting with SQSTM1. (A) ACLY with Lys63-linked poly-ubiquitin chains were stabilized after inhibition of autophagy. Granulosa cells were co-transfected with or without Atg5 shRNA, and the cell lysates were immunoprecipitated to affinity isolate the endogenous ACLY protein, and the polyubiquitinated linkage site was detected using indicated antibodies. Each lane corresponds to an independent biological sample. (B) ACLY is preferentially tagged by Lys63-linked poly-ubiquitin chains. HEK293T cells were co-transfected with plasmids expressing MYC-ACLY together with either wild-type, Lys63, or Lys48 HA-Ub. (C) Ubiquitination levels of ACLY after SQSTM1 overexpression. HEK293T cells were transfected with MYC-ACLY along with the indicated plasmids. 24 h after transfection, immunoprecipitation and immunoblot analysis were performed with the indicated antibodies. (D) SQSTM1 targets K63-linked ACLY for degradation by its UBA domain. Granulosa cells were transfected with MYC-tagged ACLY and FLAG-tagged wild-type SQSTM1 or its UBA domain deletion variant. (E) SQSTM1 interacts with ACLY. Endogenous SQSTM1 was immunoprecipitated with an antibody against SQSTM1. ACLY was detected by western blotting. (F) ACLY is stabilized in sqstm1 knockout cells. Control or CRISPR/Cas9-mediated sqstm1 KO granulosa cells were then treated with CHX for the indicated time. The percentage of remaining ACLY protein was calculated from the intensity of CHX treatment immunoblots, measured in three biological replicates.
