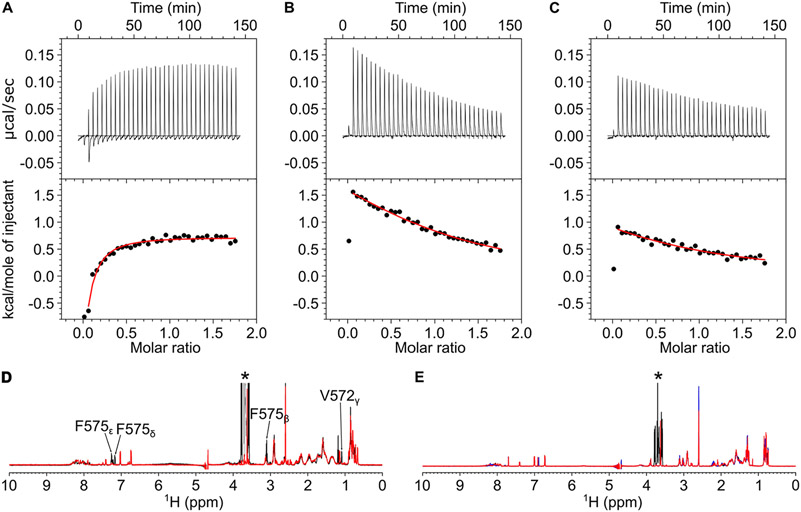
Figure 7.
CaM binding to CNGB1 linchpin mutants (CaM1F575E, CaM2L1132E, and CaM2L1136E). ITC isotherms of the CaM N-lobe binding to CaM1F575E (A) and CaM C-lobe binding to CaM2L1132E (B) and CaM2L1136E (C). Each isotherm was fit to a one-site model, and the binding parameters (ΔH and Kd) are given in Table 1. The experimental conditions are provided in the Materials and Methods section. NMR spectra of (D) CaM1WT (black) and CaM1F575E (red) and (E) CaM2WT (black), CaM2L1132E (red), and CaM2L1136E (blue) suggest that CaM1 and CaM2 both form an α-helix and the mutants are structurally intact.