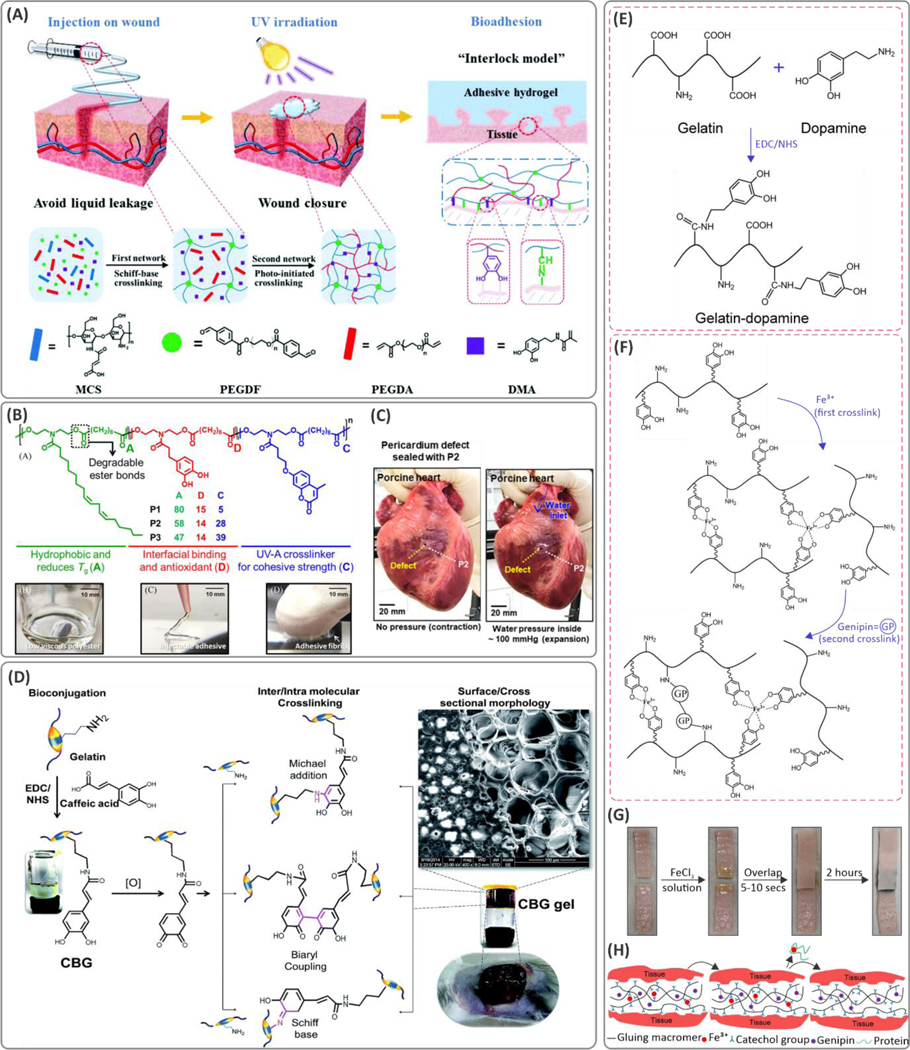
Figure 12. Examples of copolymerization of catechol-containing monomers, and conjugating catechol groups to the hydrogel polymer backbones for enhancing adhesion.
(A) Schematic of the fit-to-shape photocurable sealants and underlying mechanisms of crosslinking and adhesion to the tissue in wound-closure applications. The hydrogel is composed of PEGDF, maleic-modified chitosan (MCS), dopamine methacrylate (DMA), and poly(ethylene glycol)diacrylate (PEGDA) components. Reproduced with permission from ref 18. Copyright 2019, Royal Society of Chemistry. (B) Molecular design of statistical co-polymer adhesives consisting of hydrophobic, interfacial bonding region, and crosslinking portions. (C) Application of the hydrogel for sealing incision and defects on the myocardium tissue. Reprinted with permission from ref 286. Copyright 2019 American Chemical Society. (D) Carbodiimide coupling of the caffeic acid to the gelatin backbone as a wound dressing material. The caffeic-acid-bioconjugated gel (CBG) was crosslinked through Michael addition, biaryl coupling, as well as Schiff base formation mechanisms. Reproduced with permission from ref 289. Copyright 2015, Royal Society of Chemistry. (E) Chemical conjugation of the dopamine to the gelatin backbone using carbodiimide chemistry. (F) Mechanism of dual ionic and covalent crosslinking of the catechol conjugated gelatin using Fe3+ ions and genipin. (G) The lap shear adhesion test and (H) the molecular mechanisms of adhesion to the tissue substrates. Reproduced with permission from ref 219. Copyright 2016, Elsevier.