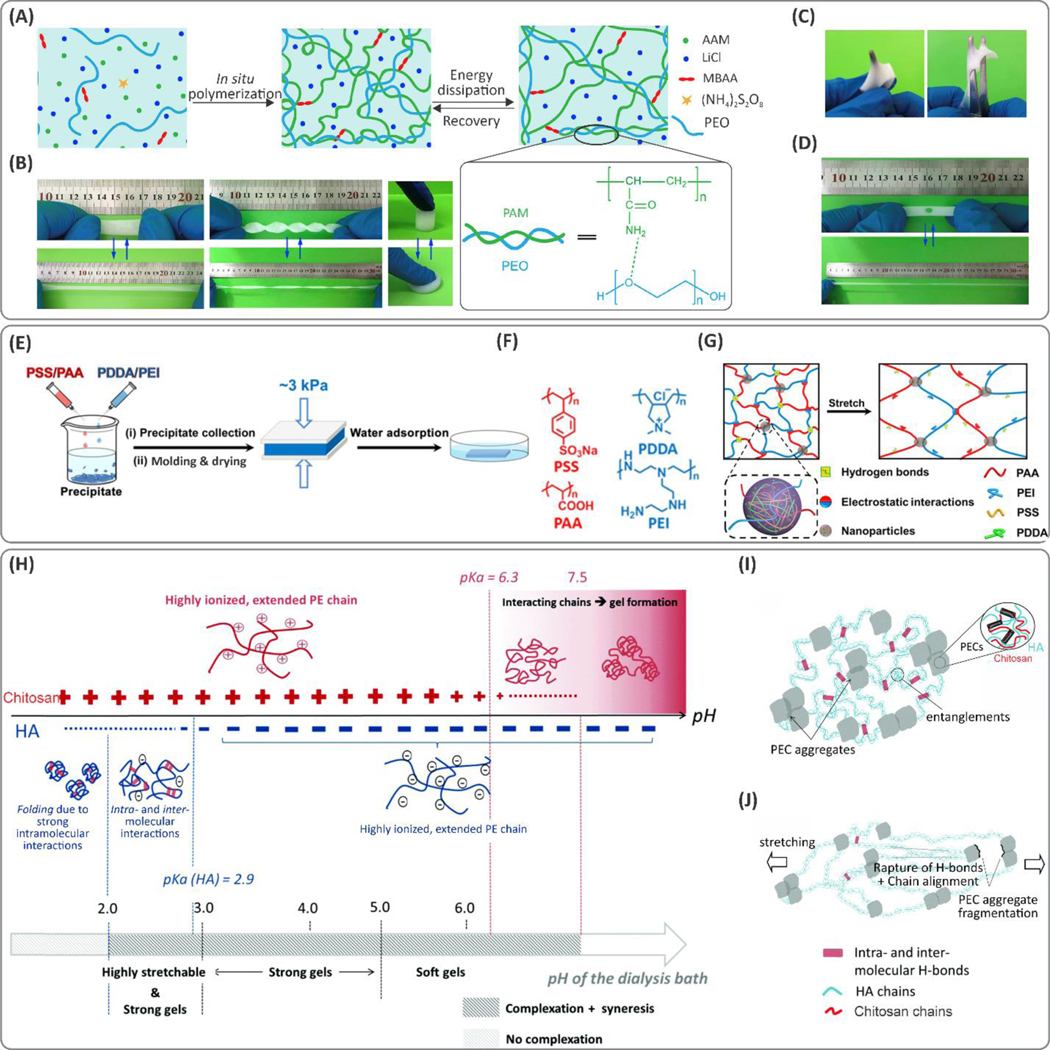
Figure 2. Examples of interpenetrating hydrogel networks (IPN) with improved toughness.
(A) Schematic of components and interactions in an IPN of poly(acrylamide) (PAM)/poly(ethylene oxide) (PEO) hydrogel crosslinked by N,N’-methylene-bis(acrylamide) (MBAA) toughened due to the synergy between hydrogen-bonding and covalent networks. (B) Hydrogel resistance against tensile, compressive, and torsion deformations. The hydrogels were able to stretch by ~8.8×. (C) Hydrogels showed anti-puncturing characteristics. (D) The punctured hydrogels still maintained their stretchability. Reproduced with permission from ref 57. Copyright 2019, Elsevier. (E) Schematic of processing poly(diallyldimethylammonium chloride) (PDDA)-poly(sodium 4-styrenesulfonate) (PSS)/branched poly(ethylenimine) (PEI)-poly(acrylic acid) (PAA) hydrogels. (F) Chemical structure of PSS, PAA, PEI, and PDDA components. (G) Toughening mechanism of the hydrogels: the effect of electrostatic interactions and hydrogen bonding. Reprinted with permission from ref 59. Copyright 2019 American Chemical Society. (H) Effect of pH on the conformation of polyelectrolytes (PE). At low pH, chitosan is ionized resulting in polyelectrolyte repulsion. Hyaluronic acid (HA), on the other hand, tends to fold due to intramolecular interactions. As the pH increases (above pKa of chitosan), a random coil conformation is formed by chitosan. Any further increase above pKa of HA leads to its extended conformation. Schematic of molecular reformation of the chains before (I) and after (J) the application of stretching loads. The dissipative process stems from the interchain hydrogen bonding and crosslinking through the polyelectrolyte complex (PEC) aggregates and hydrogen bonding. Reproduced with permission from ref 60. Copyright 2017, Royal Society of Chemistry.