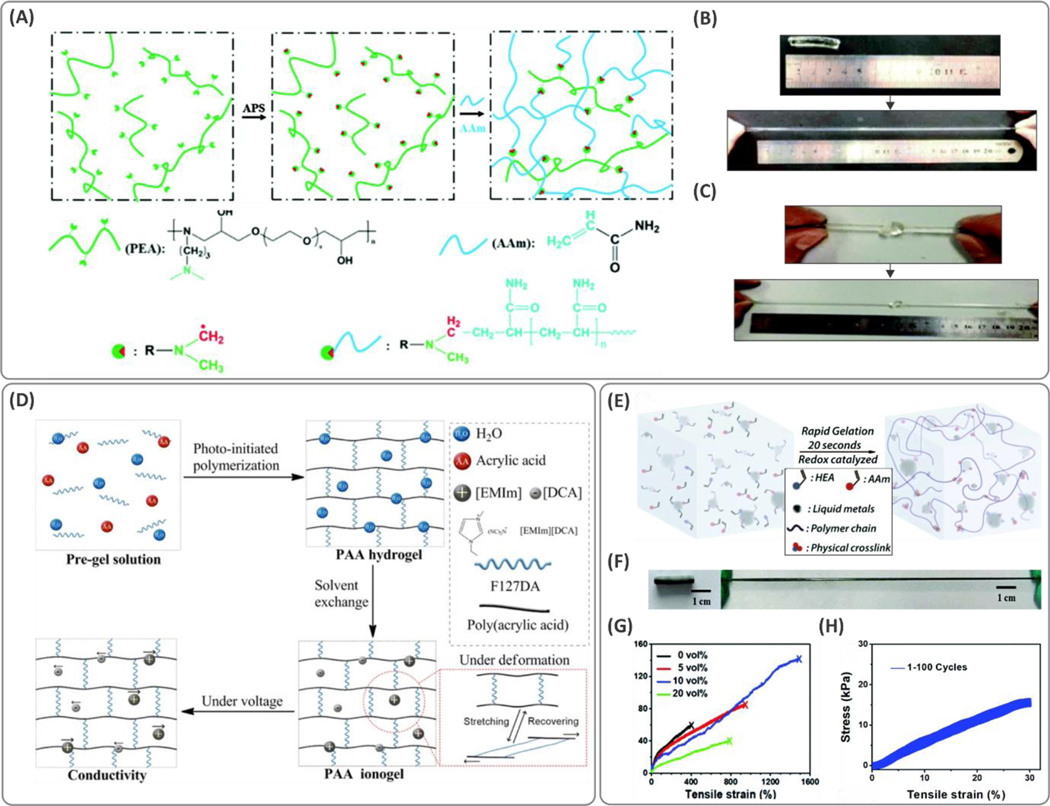
Figure 4. Creating tougher hydrogels through functionalization of the crosslinker and polymeric backbone.
(A) Functionalization of polyetheramine (PEA) with tertiary amines allowed the polymeric backbone to act as both the initiator and crosslinker during the radical polymerization in the presence of poly(acrylamide) (PAM) network. (B, C) Tough and stretchable hydrogels were obtained with stretchability of up to 2000%. Reproduced with permission from ref 75. Copyright 2016, Royal Society of Chemistry. (D) Schematic illustration of the synthesis and deformation mechanism of poly(acrylic acid) (PAA) hydrogels crosslinked with Pluronic F127 (F127DA). Ionogels were fabricated by adding ionic liquid 1-ethyl-3-methylimidazolium dicyanamide ([EMIm][DCA]) through a solvent exchange process. The hydrogel showed high fatigue resistance for strains of up to 850%. Reprinted with permission from ref 66. Copyright 2019 American Chemical Society. (E) Rapid synthesis procedure of the liquid metal-based hydrogels acrylamide (AAm) and 2-hydroxyethyl acrylate (HEA) formed via a redox catalyzed reaction. (F) Elasticity of the hydrogel and (G) the stress-strain curves showing stretchability at the optimized concentration of liquid metal (up to ~1500%). (H) Cyclic stress-strain curves of the hydrogel for 100 cycles. Reproduced with permission from ref 79. Copyright 2019, Royal Society of Chemistry.