Abstract
Uric acid produced by guanine deaminase (GDA) is involved in photoaging and hyperpigmentation. Reactive oxygen species (ROS) generated by uric acid plays a role in photoaging. However, the mechanism by which uric acid stimulates melanogenesis in GDA-overexpressing keratinocytes is unclear. Keratinocyte-derived paracrine factors have been identified as important mechanisms of ultraviolet-induced melanogenesis. Therefore, the role of paracrine melanogenic growth factors in GDA-induced hypermelanosis mediated by uric acid was examined. The relationships between ROS and these growth factors were examined. Primary cultured normal keratinocytes overexpressed with wild type or mutant GDA and those treated with xanthine or uric acid in the presence or absence of allopurinol, H2O2, or N-acetylcysteine (NAC) were used in this study. Intracellular and extracellular bFGF and SCF levels were increased in keratinocytes by wild type, but not by loss-of-function mutants of GDA overexpression. Culture supernatants from GDA-overexpressing keratinocytes stimulated melanogenesis, which was restored by anti-bFGF and anti-SCF antibodies. Allopurinol treatment reduced the expression levels of bFGF and SCF in both GDA-overexpressing and normal keratinocytes exposed to exogenous xanthine; the exogenous uric acid increased their expression levels. H2O2-stimulated tyrosinase expression and melanogenesis were restored by NAC pretreatment. However, H2O2 or NAC did not upregulate or downregulate bFGF or SCF, respectively. Overall, uric acid could be involved in melanogenesis induced by GDA overexpression in keratinocytes via bFGF and SCF upregulation not via ROS generation.
Keywords: Uric acid, GDA-induced melanogenesis, Keratinocyte-derived melanogenic growth factors
INTRODUCTION
Seborrheic keratosis frequently occurs in sun-exposed areas on the skin of the elderly. Based on the predictable role of photoaging in seborrheic keratosis, it is found that upregulated guanine deaminase (GDA) is involved in keratinocyte senescence induced by ultraviolet (UV) radiation. Reactive oxygen species (ROS) generated by a metabolic end product such as uric acid have been found to play a role in keratinocyte senescence (Cheong and Lee, 2020). The role of skin aging in melasma has also been identified (Kim et al., 2016, 2017; Passeron and Picardo, 2018; Kwon et al., 2019). UV is also the most well-known factor involved in skin hypermelanosis (Miyamura et al., 2007; Guida et al., 2021). Chronic UV exposure has been considered as one of main causes for the development of melasma (Lee, 2015; Passeron and Picardo, 2018; Kwon et al., 2019), a representative skin hyperpigmented skin disorder. Seborrheic keratosis frequently displays pigments, with keratinocytes containing greater amount of melanin, similar to melasma. Based on these common features between seborrheic keratosis and melasma, GDA was found to be upregulated in melasma (Supplementary Fig. 1) as shown in published reports(Chung et al., 2014; Jung et al., 2020). The role of uric acid released from GDA-overexpressing keratinocytes in hyperpigmentation has also been reported (Cheong et al., 2021).
Photoaging is often accompanied by skin hyperpigmentation. As a mechanism for UV-induced melanogenesis, paracrine factors derived from keratinocytes have been established (López et al., 2015; Swope et al., 2020; Takano et al., 2020). Increased levels of endothelin-1 (ET-1) have also been presented as a potential mechanism of hyperpigmentation in seborrheic keratosis (Takenaka et al., 2013). Roles of uric acid in both melanogenesis and photoaging (Cheong and Lee, 2020; Cheong et al., 2021) cast doubt on uric acid as a common mechanism for UV-induced melanogenesis and photoaging. ROS generated by uric acid are involved in photoaging (Cheong and Lee, 2020). However, the mechanism by which uric acid from GDA-overexpressing keratinocytes stimulates melanogenesis is uncertain; this could be through ROS or via paracrine melanogenic growth factors, such as ET-1, stem cell factor (SCF), and basic fibroblast growth factor (bFGF) derived from the keratinocytes (Hachiya et al., 2004; Brenner et al., 2005; Stanisz et al., 2012; Terazawa and Imokawa, 2018). Thus, the objective of this study was to examine whether these paracrine melanogenic growth factors played a role in GDA-induced hypermelanosis mediated by uric acid. Relations of ROS with these growth factors were also examined.
MATERIALS AND METHODS
Normal human epidermal cell culture
Adult skin specimens obtained from Cesarean sections and circumcisions were used for establishing cells for cell culture. The epidermis was separated from the dermis. Suspensions of individual epidermal cells were prepared. Keratinocytes were suspended in EpiLife Medium (Invitrogen, Carlsbad, CA, USA) supplemented with bovine pituitary extract (BPE), bovine insulin (BI), hydrocortisone, human epidermal growth factor, and bovine transferrin (BT) (Invitrogen). Melanocytes were suspended in Medium 254 (Invitrogen) supplemented with BPE, fetal bovine serum, BI, hydrocortisone, bFGF, BT, heparin, and phorbol 12-myristate 13-acetate (Invitrogen). In case of keratinocytes, passages from 3 to 5 were used for experiments. For coculture of keratinocytes and melanocytes, keratinocytes were seeded at 2×105 cells/well to six-well plates and incubated for 24 h. Keratinocytes were transfected with indicated genes. Four hours later, 1×105 melanocytes/well in 2 mL of EpiLife media without any supplement were added to transfected cells. After 24 h or 48 h, cells and the supernatants were harvested for next experiments. In case of supernatant culture as a conditioned media, 4 h after transfection of keratinocytes, 2 mL of EpiLife media without any supplement were added to transfected cells. At the same time, 1×105 cells/well melanocytes were seeded to another six-well plates. At 24 or 48 h later, media of melanocyte were changed to supernatants obtained from transfected keratinocytes. One day later, cells and supernatants were collected for the next step. For neutralization of bFGF or SCF, neutralizing antibodies to bFGF (Millipore, Billerica, MA, USA) or SCF (Abcam, Boston, MA, USA) were added to keratinocytes transfected with GDA. All collected cells and supernatants were used for Western blot analysis, immunohistochemistry, and ELISA.
Overexpression of GDA and GDA mutant constructs
To construct GDA, amplified PCR products were inserted to pCMV vector. GDA mutants with decreased guanine deaminase activity were constructed using a previously described method (Akum et al., 2004) with some modification. Briefly, the zinc binding domain (amino acid 76-84) or collapsing response mediator protein (CRMP) homology domain (amino acids 350-402), which could attenuate guanine deaminase activity, was deleted respectively or both. These mutants were named GDA∆(76-84) and GDA∆(76-84 and 350-402). For overexpression of GDA and GDA mutants, transfection of cells was carried out using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Cells were used for experiments at 24 or 48 h after transfection.
Xanthine, uric acid, and allopurinol treatment
One day following keratinocyte seeding (2×105 keratinocytes/well), cells were treated with appropriate concentrations (10 and 20 µM) of xanthine with or without allopurinol (50 µM) or uric acid (5 and 10 µM) (Sigma-Aldrich, St. Louis, MO, USA) for 24 to 48 h. Cells and supernatants were harvested for subsequent experiments.
H2O2 and NAC treatment
At 24 h after GDA transfection, media of keratinocytes were changed without any supplement. N-acetyl-L-cysteine (NAC, 5 mM) (Sigma-Aldrich) or H2O2 (100 uM) were added to cells. At 24 and 48 h later, harvested cells were subjected to Western blot analysis for protein expression.
Cell viability test
To measure cell viability, cells were incubated with MTT for 4 h. Precipitated formazan was dissolved in dimethyl sulfoxide (DMSO). The optical density was measured at 570 nm with background subtraction at 630 nm using a spectrophotometer.
Western blot analysis and ELISA
Equal amounts of extracted proteins were resolved and transferred to nitrocellulose membranes. These membranes were incubated with antibodies to GDA, tyrosinase, SCF, xanthine oxidase (mouse monoclonal; Santa Cruz Biotechnology, Dallas, TX, USA), MITF, bFGF, p-CREB, CREB (rabbit polyclonal; cell signaling technology, Beverly, MA, USA), and β-actin (mouse monoclonal; Sigma-Aldrich). After incubating with appropriate anti-mouse or anti-rabbit horseradish peroxidase-conjugated antibodies (Thermo Fisher Scientific, Waltham, MA, USA) or with anti-goat horseradish peroxidase-conjugated antibody (Santa Cruz Biotechnology), enhanced chemiluminescence solution (Thermo Fisher Scientific) was applied and signals were captured with an image reader (LAS-3000; Fuji Photo Film, Tokyo, Japan). Protein bands were then analyzed by densitometry. Concentrations of bFGF (R&D Systems, Minneapolis, MN, USA) and SCF (Abcam) in culture supernatants were measured using ELISA kits according to the manufacturer’s instructions.
Immunohistochemistry
After deparaffinization and rehydration, sections were preincubated with 3% bovine serum albumin. These sections were reacted sequentially with anti-GDA antibody, 1:200 Alexa Fluor-labeled goat anti-mouse IgG (488; Molecular Probes, Eugene, OR, USA), anti-bFGF (BD biosciences, San Jose, CA, USA), or anti-SCF (Santa Cruz Biotechnology) and Alexa Fluor-labeled goat anti-mouse IgG (594; Molecular Probes). Nuclei were counterstained with Hoechst 33258 (Sigma-Aldrich). Fluorescence images were evaluated using an image analysis system (Dp Manager 2.1; Olympus Optical Co., Tokyo, Japan) and Wright Cell Imaging Facility (WCIF) ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Statistical analyses of experimental data were performed using Student’s t-test. Results are expressed as means ± SD. A p-value of less than 0.05 was considered significant.
RESULTS
Expression levels of bFGF and SCF in keratinocytes are increased by GDA overexpression
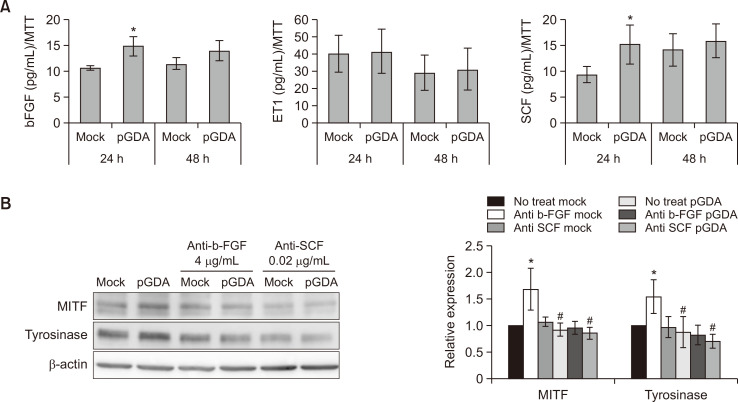
To examine a potential role of paracrine melanogenic growth factors from GDA-overexpressing keratinocytes in melanogenesis, expression levels of growth factors derived from keratinocytes with or without GDA overexpression were checked. Effect of culture supernatant on melanogenesis was also examined. ELISA results showed that concentrations of bFGF and SCF were increased in culture supernatants obtained from viable GDA-overexpressing keratinocytes (Fig. 1A). Western blot analysis showed that relative expression levels of microphthalmia-associated transcription factor (MITF), the most critical transcription factor required for melanogenesis (Lee and Noh, 2013), and tyrosinase proteins were increased in melanocytes cultured with supernatants obtained from GDA-overexpressing keratinocytes (Fig. 1B). These increases of MITF and tyrosinase proteins were restored by anti-bFGF and SCF antibodies (Fig. 1B).
Fig. 1.
Expression levels of bFGF and SCF are increased in keratinocytes with GDA overexpression. (A) Concentrations of bFGF, SCF, and ET-1 in primary cultured human keratinocytes with or without GDA overexpression based on ELISA. (B) Western blot analysis of relative ratios of protein levels of MITF and tyrosinase in melanocytes treated with culture supernatants from GDA-overexpressing keratinocytes in the presence or absence of anti-bFGF and anti-SCF antibodies. β-Actin was used as an internal control for Western blot analysis. Data are as present means ± SD of four independent experiments. *p<0.05 vs. control keratinocytes, #p<0.05 vs. non-treated GDA-overexpressing keratinocytes.
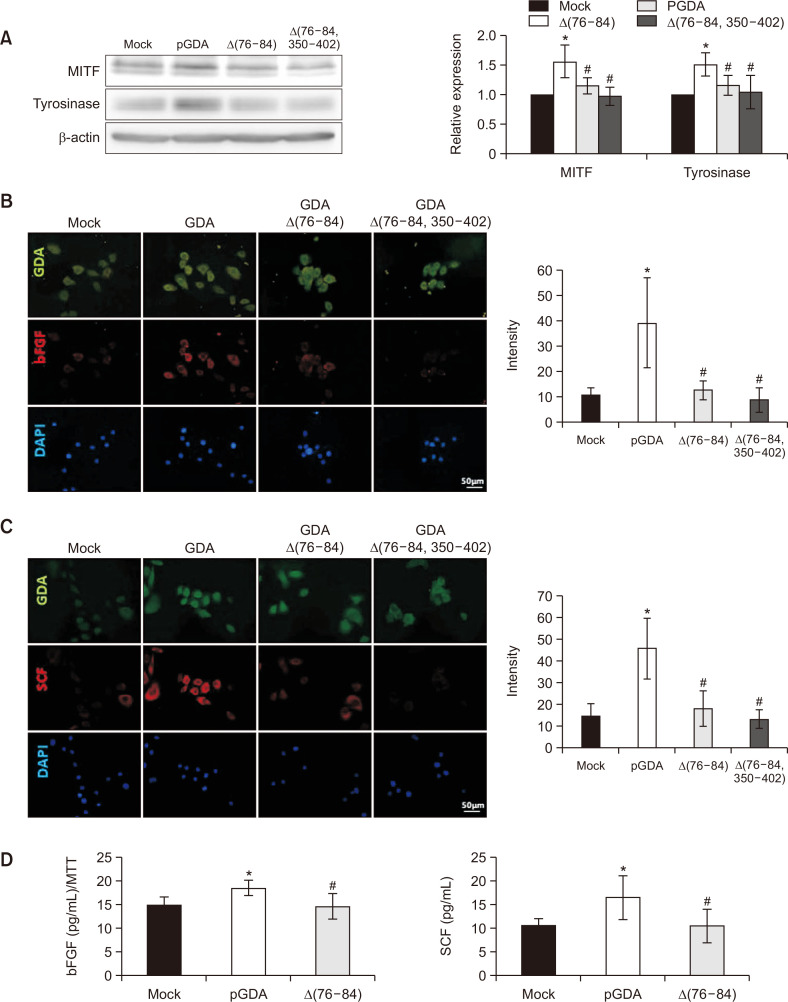
Loss-of-function mutants of GDA do not increase melanogenesis or concentrations of bFGF or SCF
GDAΔ(76-84) and GDAΔ(76-84 and 350-402) were loss-of-function mutants of GDA (Cheong and Lee, 2020). Overexpression of these mutants of GDA did not stimulate relative expression levels of MITF or tyrosinase proteins (Fig. 2A). Immunofluorescence staining intensities against anti-bFGF antibody were weaker in keratinocytes with overexpression of GDAΔ(76-84) or GDAΔ(76-84 and 350-402) compared to those with wild type GDA overexpression (Fig. 2B). Staining intensities against anti-SCF antibody did not increase in keratinocytes with either GDAΔ(76-84) or GDAΔ(76-84 and 350-402) overexpression either (Fig. 2C). Concentrations of bFGF and SCF proteins were not increased in culture supernatants from keratinocytes with GDAΔ(75-84) overexpression (Fig. 2D).
Fig. 2.
Loss-of-function mutants of GDA do not increase melanogenesis or concentrations of bFGF or SCF. (A) Western blot analysis for relative ratios of protein levels of MITF and tyrosinase in melanocytes treated with culture supernatants from keratinocytes with wild-type or loss-of-function mutants of GDA overexpression. β-Actin was used as an internal control. (B, C) Immunofluorescence staining with anti-GDA and anti-bFGF (B) or with anti-GDA and anti-SCF antibodies (C) in keratinocytes with overexpression of wild-type GDA or loss-of-function mutants of GDA (Bar=0.05 mm). Nuclei were counter-stained with Hoechst 33258. Intensities of immunofluorescence staining were measured using a Wright Cell Imaging Facility ImageJ software. (D) ELISA for concentrations of bFGF and SCF in primary cultured keratinocytes with or without loss-of-function mutant of GDA overexpression. Data are presented as means ± SD of four independent experiments. *p<0.05 vs. control keratinocytes, #p<0.05 vs. non-treated GDA-overexpressing keratinocytes.
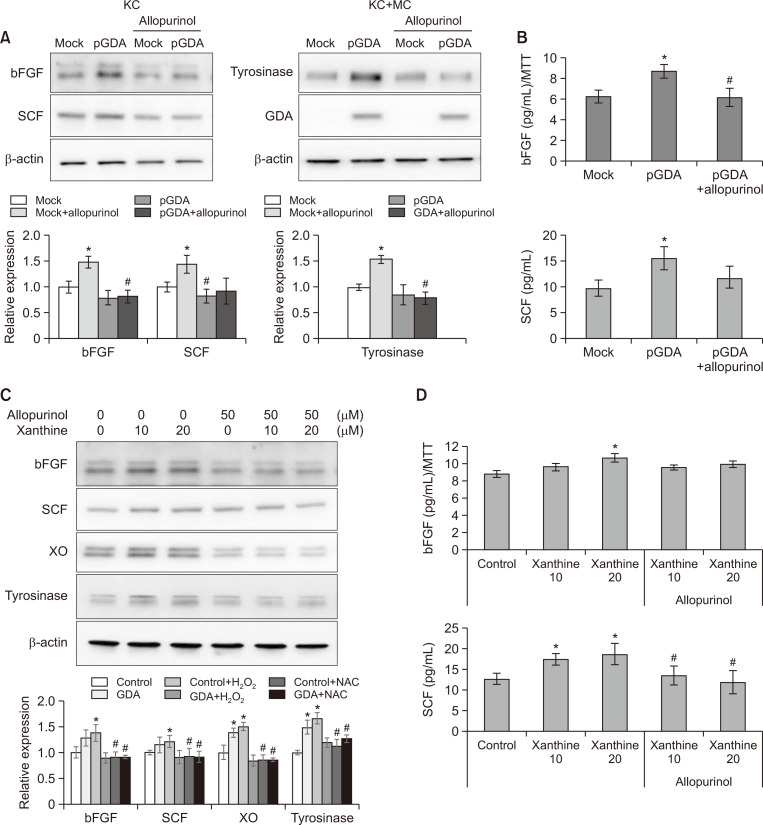
GDA-induced bFGF and SCF upregulation is restored by allopurinol
Overexpression of loss-of-function mutants of GDA significantly reduced uric acid, a metabolic end product of guanine, compared with wild type overexpression (Cheong and Lee, 2020). Allopurinol inhibits xanthine oxidoreductase (XO), an enzyme involved in metabolizing xanthine into uric acid. Thus, expression levels of bFGF and SCF were examined in GDA-overexpressing keratinocytes and normal keratinocytes after treatment with xanthine in the presence or absence of allopurinol. Increased expression levels of bFGF and SCF in GDA-overexpressing keratinocytes were reduced by allopurinol (Fig. 3A). ELISA results also showed increased bFGF and SCF concentrations in culture supernatants of GDA-overexpressing keratinocytes. These increases were restored by allopurinol (Fig. 3B). Allopurinol also decreased expression levels (Fig. 3C) and released concentrations (Fig. 3D) of bFGF and SCF from normal keratinocytes treated with exogenous xanthine.
Fig. 3.
GDA-induced bFGF and SCF upregulation is restored by allopurinol. (A, B) Western blot analysis for relative ratios (A) and ELISA for concentrations (B) of bFGF and SCF protein levels in keratinocytes with or without GDA overexpression in the presence or absence of allopurinol. *p<0.05 vs. control keratinocytes, #p<0.05 vs. non-treated GDA-overexpressing keratinocytes. (C, D) Western blot analysis for relative ratios (C) and ELISA for concentrations (D) of bFGF and SCF protein levels in keratinocytes treated with or without different concentrations of xanthine and fixed concentration of allopurinol. *p<0.05 vs. control keratinocytes, #p<0.05 vs. keratinocytes applied with corresponding concentration of xanthine. β-Actin was used as an internal control for Western blot analysis. Data are presented as means ± SD of four independent experiments.
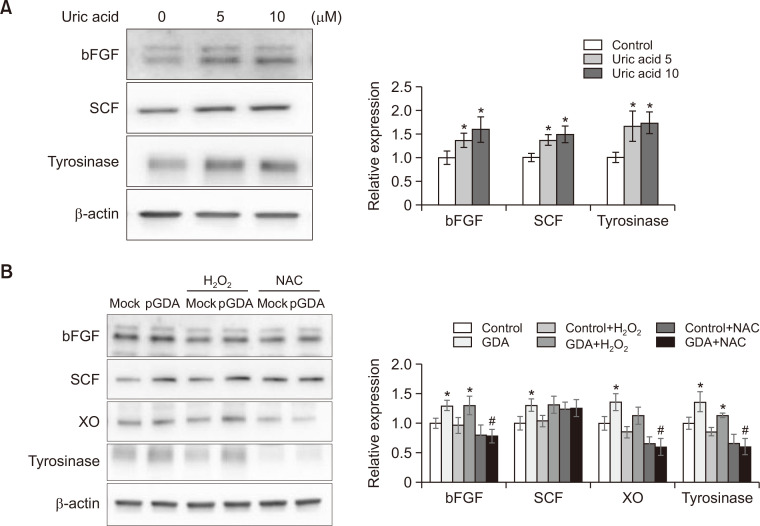
Uric acid is involved in bFGF and SCF upregulation not via ROS generation
Exogenous xanthine can increase uric acid generation and release, similar to GDA overexpression (Cheong and Lee, 2020), suggesting that uric acid could be involved in upregulation of bFGF and SCF. However, allopurinol is an inhibitor of XO, which generates ROS (Furuhashi, 2020; Liu et al., 2021). Because uric acid also generates ROS (Cheong and Lee, 2020), whether uric acid can upregulate growth factors via ROS generation remains unclear. Thus, expression levels of bFGF and SCF in normal keratinocytes treated with uric acid and in GDA-overexpressing keratinocytes in the presence or absence of H2O2 and N-acetylcysteine (NAC) were examined. Uric acid increased expression levels of bFGF and SCF in a dose-dependent manner. It also increased levels of tyrosinase (Fig. 4A). H2O2 treatment also stimulated expression levels of tyrosinase, whereas NAC pretreatment reduced tyrosinase levels. However, either H2O2 or NAC failed to increase expression levels of bFGF or SCF in GDA-overexpressing keratinocytes (Fig. 4B).
Fig. 4.
Uric acid is involved in bFGF and SCF upregulation not via ROS generation. (A, B) Western blot analysis for relative ratios of bFGF and SCF protein levels in normal keratinocytes treated with different concentrations of uric acid (A) in GDA-overexpressing keratinocytes in the presence or absence of H2O2 or NAC (B). β-Actin was used as an internal control for Western blot analysis. Data are presented as means ± SD of four independent experiments. *p<0.05 vs. control keratinocytes, #p<0.05 vs. non-treated GDA-overexpressing keratinocytes.
DISCUSSION
GDA overexpression upregulated keratinocyte-derived melanogenic growth factors such as bFGF and SCF (Fig. 1A). Expression levels of MITF and tyrosinase proteins upregulated by GDA-overexpressing keratinocytes were restored by neutralizing antibodies against bFGF and SCF (Fig. 1B). These results suggest a role of GDA-overexpressing keratinocytes in melanogenesis via bFGF and SCF upregulation. In addition, overexpression of loss-of-function GDA mutants did not increase expression levels of MITF or tyrosinase proteins (Fig. 2A). They did not increase the expression levels of bFGF or SCF proteins as well (Fig. 2B-2D), supporting the idea that upregulation of bFGF and SCF may be associated with melanogenesis in GDA-overexpressing keratinocytes. Previously, keratinocyte-derived paracrine factors have been reported in connection with seborrheic keratosis or GDA upregulation; increased levels of ET-1 and endothelin B receptor have been considered as potential mechanisms of hyperpigmentation in seborrheic keratosis (Manaka et al., 2001; Takenaka et al., 2013). Skin pigmentation caused by UV exposure and chronological aging can be regulated by p53 (Box and Terzian, 2008; Yardman-Frank and Fisher, 2021; Hoyos et al., 2022) via melanogenic cytokines, such as SCF and ET-1 (Murase et al., 2009; Hyter et al., 2013). Our previous result of p53 upregulation in keratinocytes by GDA overexpression (Cheong and Lee, 2020) suggests that SCF and ET-1 could participate in GDA-induced hyperpigmentation. Although the role of ET-1 was insignificant in this study, SCF and ET-1 were upregulated when GDA was overexpressed (Jung et al., 2020). On the other hand, there are no data on the role of bFGF in GDA-induced melanogenesis. However, our results (Fig. 1A, 1B) showed a role of bFGF in GDA-induced melanogenesis. This was expected because GDA-induced melanogenesis is closely related to hyperpigmentation caused by UV exposure (Gilchrest et al., 1996; Brenner et al., 2005; Yamaguchi and Hearing, 2009).
Loss-of-function mutants of GDA, which reduced uric acid production (Cheong and Lee, 2020), did not increase intracellular or extracellular bFGF or SCF proteins (Fig. 2B-2D). Allopurinol treatment may inhibit the production of uric acid as a metabolic end product (Vickneson and George, 2020; Orhan and Deniz, 2021). Further, allopurinol did not increase the production or release of bFGF or SCF in GDA-overexpressing or normal keratinocytes to which exogenous xanthine was applied (Fig. 3A-3D). These results suggest that uric acid could be involved in melanogenesis via generation of bFGF and SCF. In fact, increased expression levels of bFGF and SCF by exogenous uric acid (Fig. 4A) supported the role of uric acid in upregulating of bFGF and SCF.
In addition to inhibition of uric acid production, allopurinol as an XO inhibitor could obstruct ROS generation by XO (Furuhashi, 2020; Liu et al., 2021). Exogenous xanthine also stimulated XO generation in keratinocytes (data not shown). Uric acid was involved in photoaging via ROS generation (Cheong and Lee, 2020). These results suggest the possible roles of ROS in melanogenesis and photoaging (Maranduca et al., 2019; Papaccio et al., 2022). It might be a mechanism of hyperpigmentation associated with photoaging. In fact, H2O2 treatment increased expression levels of tyrosinase, whereas NAC pretreatment decreased their levels in normal keratinocytes with GDA overexpression (Fig. 4B). However, expression levels of bFGF and SCF were not increased with increases of tyrosinase by H2O2 or NAC (Fig. 4B), indicating that bFGF and SCF upregulation did not go through ROS generation (Fig. 5).
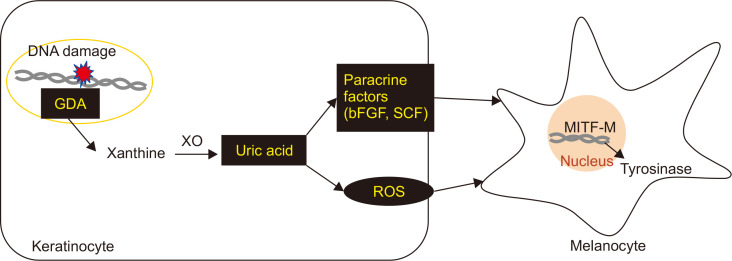
Fig. 5.
Schematic view for melanogenesis induced by uric acid upregulated in GDA-overexpressing keratinocytes. Uric acid produced by GDA upregulation in keratinocytes could enhance MITF and tyrosinase expression via bFGF and SCF production without going through ROS generation.
In summary, uric acid produced by GDA upregulation in keratinocytes could stimulate melanogenesis via bFGF and SCF production not via ROS generation.
ACKNOWLEDGMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HP20C0131).
Footnotes
REFERENCES
- Akum B. F., Chen M., Gunderson S. I., Riefler G. M., Scerri-Hansen M. M., Firestein B. L. Cypin regulates dendrite patterning in hippocampal neurons by promoting microtubule assembly. Nat. Neurosci. 2004;7:145–152. doi: 10.1038/nn1179. [DOI] [PubMed] [Google Scholar]
- Box N. F., Terzian T. The role of p53 in pigmentation, tanning and melanoma. Pigment Cell Melanoma Res. 2008;21:525–533. doi: 10.1111/j.1755-148X.2008.00495.x. [DOI] [PubMed] [Google Scholar]
- Brenner M., Degitz K., Besch R., Berking C. Differential expression of melanoma-associated growth factors in keratinocytes and fibroblasts by ultraviolet A and ultraviolet B radiation. Br. J. Dermatol. 2005;153:733–739. doi: 10.1111/j.1365-2133.2005.06780.x. [DOI] [PubMed] [Google Scholar]
- Cheong K. A., Kil I. S., Ko H. W., Lee A.-Y. Upregulated guanine deaminase is involved in hyperpigmentation of seborrheic keratosis via uric acid release. Int. J. Mol. Sci. 2021;22:12501. doi: 10.3390/ijms222212501.79ddfd26e3474fd5bffd9533f7ba9a9e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong K. A., Lee A.-Y. Guanine deaminase stimulates ultraviolet-induced keratinocyte senescence in seborrhoeic keratosis via guanine metabolites. Acta Derm.-Venereol. 2020;100:adv00109. doi: 10.2340/00015555-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung B. Y., Noh T. K., Yang S. H., Kim I. H., Lee M. W., Yoon T. J., Chang S. E. Gene expression profiling in melasma in Korean women. Dermatology. 2014;229:333–342. doi: 10.1159/000365080. [DOI] [PubMed] [Google Scholar]
- Furuhashi M. New insights into purine metabolism in metabolic diseases: role of xanthine oxidoreductase activity. Am. J. Physiol. Endocrinol. Metab. 2020;319:E827–E834. doi: 10.1152/ajpendo.00378.2020. [DOI] [PubMed] [Google Scholar]
- Gilchrest B. A., Park H.-Y., Eller M. S., Yaar M. Mechanisms of ultraviolet light-induced pigmentation. Photochem. Photobiol. 1996;63:1–10. doi: 10.1111/j.1751-1097.1996.tb02988.x. [DOI] [PubMed] [Google Scholar]
- Guida S., Guida G., Goding C. R. MC1R functions, expression, and implications for targeted therapy. J. Invest. Dermatol. 2021;142:293–302.e1. doi: 10.1016/j.jid.2021.06.018. [DOI] [PubMed] [Google Scholar]
- Hachiya A., Kobayashi A., Yoshida Y., Kitahara T., Takema Y., Imokawa G. Biphasic expression of two paracrine melanogenic cytokines, stem cell factor and endothelin-1, in ultraviolet B-induced human melanogenesis. Am. J. Pathol. 2004;165:2099–2109. doi: 10.1016/S0002-9440(10)63260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyos D., Greenbaum B., Levine A. J. The genotypes and phenotypes of missense mutations in the proline domain of the p53 protein. Cell Death Differ. 2022;29:938–945. doi: 10.1038/s41418-022-00980-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyter S., Coleman D. J., Ganguli-Indra G., Merrill G. F., Ma S., Yanagisawa M., Indra A. K. Endothelin-1 is a transcriptional target of p53 in epidermal keratinocytes and regulates ultraviolet-induced melanocyte homeostasis. Pigment Cell Melanoma Res. 2013;26:247–258. doi: 10.1111/pcmr.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J. M., Noh T. K., Jo S. Y., Kim S. Y., Song Y., Kim Y.-H., Chang S. E. Guanine deaminase in human epidermal keratinocytes contributes to skin pigmentation. Molecules. 2020;25:2637. doi: 10.3390/molecules25112637.9ba4120859fa49b7a048c06e76853036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N. H., Choi S. H., Lee T. R., Lee C. H., Lee A. Y. Cadherin 11 involved in basement membrane damage and dermal changes in melasma. Acta Derm.-Venereol. 2016;96:635–640. doi: 10.2340/00015555-2315. [DOI] [PubMed] [Google Scholar]
- Kim N. H., Choi S. H., Yi N., Lee T. R., Lee A. Y. Arginase-2, a miR-1299 target, enhances pigmentation in melasma by reducing melanosome degradation via senescence-induced autophagy inhibition. Pigment Cell Melanoma Res. 2017;30:521–530. doi: 10.1111/pcmr.12605. [DOI] [PubMed] [Google Scholar]
- Kwon S. H., Na J. I., Choi J. Y., Park K. C. Melasma: updates and perspectives. Exp. Dermatol. 2019;28:704–708. doi: 10.1111/exd.13844. [DOI] [PubMed] [Google Scholar]
- Lee A.-Y., Noh M. The regulation of epidermal melanogenesis via cAMP and/or PKC signaling pathways: insights for the development of hypopigmenting agents. Arch. Pharm. Res. 2013;36:792–801. doi: 10.1007/s12272-013-0130-6. [DOI] [PubMed] [Google Scholar]
- Lee A. Y. Recent progress in melasma pathogenesis. Pigment Cell Melanoma Res. 2015;28:648–660. doi: 10.1111/pcmr.12404. [DOI] [PubMed] [Google Scholar]
- Liu N., Xu H., Sun Q., Yu X., Chen W., Wei H., Jiang J., Xu Y., Lu W. The role of oxidative stress in hyperuricemia and xanthine oxidoreductase (XOR) inhibitors. Oxid. Med. Cell. Longev. 2021;2021:1470380. doi: 10.1155/2021/1470380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López S., Alonso S., Garcia, de Galdeano A., Smith-Zubiaga I. Melanocytes from dark and light skin respond differently after ultraviolet B irradiation: effect of keratinocyte-conditioned medium. Photodermatol. Photoimmunol. Photomed. 2015;31:149–158. doi: 10.1111/phpp.12169. [DOI] [PubMed] [Google Scholar]
- Manaka I., Kadono S., Kawashima M., Kobayashi T., Imokawa G. The mechanism of hyperpigmentation in seborrhoeic keratosis involves the high expression of endothelin-converting enzyme-1α and TNF-α, which stimulate secretion of endothelin 1. Br. J. Dermatol. 2001;145:895–903. doi: 10.1046/j.1365-2133.2001.04521.x. [DOI] [PubMed] [Google Scholar]
- Maranduca M. A., Branisteanu D., Serban D. N., Branisteanu D. C., Stoleriu G., Manolache N., Serban I. L. Synthesis and physiological implications of melanic pigments. Oncol. Lett. 2019;17:4183–4187. doi: 10.3892/ol.2019.10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamura Y., Coelho S. G., Wolber R., Miller S. A., Wakamatsu K., Zmudzka B. Z., Ito S., Smuda C., Passeron T., Choi W. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res. 2007;20:2–13. doi: 10.1111/j.1600-0749.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- Murase D., Hachiya A., Amano Y., Ohuchi A., Kitahara T., Takema Y. The essential role of p53 in hyperpigmentation of the skin via regulation of paracrine melanogenic cytokine receptor signaling. J. Biol. Chem. 2009;284:4343–4353. doi: 10.1074/jbc.M805570200. [DOI] [PubMed] [Google Scholar]
- Orhan I. E., Deniz F. S. Natural products and extracts as xantine oxidase inhibitors - a hope for gout disease? Curr. Pharm. Des. 2021;27:143–158. doi: 10.2174/1381612826666200728144605. [DOI] [PubMed] [Google Scholar]
- Papaccio F., Arino A., Caputo S., Bellei B. Focus on the contribution of oxidative stress in skin aging. Antioxidants. 2022;11:1121. doi: 10.3390/antiox11061121.f24c8ab77bca48809e2af74c8b647f8e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passeron T., Picardo M. Melasma, a photoaging disorder. Pigment Cell Melanoma Res. 2018;31:461–465. doi: 10.1111/pcmr.12684. [DOI] [PubMed] [Google Scholar]
- Stanisz H., Stark A., Kilch T., Schwarz E. C., Müller C. S., Peinelt C., Hoth M., Niemeyer B. A., Vogt T., Bogeski I. ORAI1 Ca2+ channels control endothelin-1-induced mitogenesis and melanogenesis in primary human melanocytes. J. Invest. Dermatol. 2012;132:1443–1451. doi: 10.1038/jid.2011.478. [DOI] [PubMed] [Google Scholar]
- Swope V. B., Starner R. J., Rauck C., Abdel-Malek Z. A. Endothelin-1 and α-melanocortin have redundant effects on global genome repair in UV-irradiated human melanocytes despite distinct signaling pathways. Pigment Cell Melanoma Res. 2020;33:293–304. doi: 10.1111/pcmr.12823. [DOI] [PubMed] [Google Scholar]
- Takano K., Hachiya A., Murase D., Tanabe H., Kasamatsu S., Takahashi Y., Moriwaki S., Hase T. Quantitative changes in the secretion of exosomes from keratinocytes homeostatically regulate skin pigmentation in a paracrine manner. J. Dermatol. 2020;47:265–276. doi: 10.1111/1346-8138.15202. [DOI] [PubMed] [Google Scholar]
- Takenaka Y., Hoshino Y., Nakajima H., Hayashi N., Kawashima M., Imokawa G. Paracrine cytokine mechanisms underlying the hyperpigmentation of seborrheic keratosis in covered skin areas. J. Dermatol. 2013;40:533–542. doi: 10.1111/1346-8138.12178. [DOI] [PubMed] [Google Scholar]
- Terazawa S., Imokawa G. Signaling cascades activated by UVB in human melanocytes lead to the increased expression of melanocyte receptors, endothelin B receptor and c-KIT. Photochem. Photobiol. 2018;94:421–431. doi: 10.1111/php.12848. [DOI] [PubMed] [Google Scholar]
- Vickneson K., George J. Reactive Oxygen Species. Springer; 2020. Xanthine oxidoreductase inhibitors; pp. 205–228. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Hearing V. J. Physiological factors that regulate skin pigmentation. Biofactors. 2009;35:193–199. doi: 10.1002/biof.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardman-Frank J. M., Fisher D. E. Skin pigmentation and its control: From ultraviolet radiation to stem cells. Exp. Dermatol. 2021;30:560–571. doi: 10.1111/exd.14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.