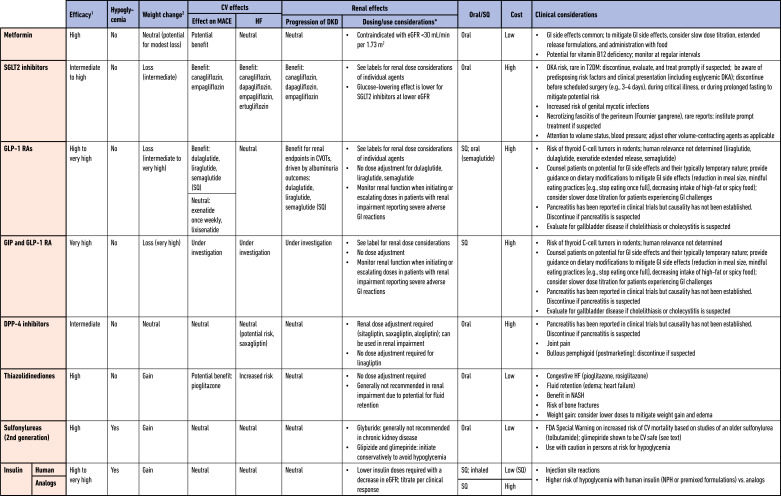
Table 9.2.
Medications for lowering glucose, summary of characteristics
CV, cardiovascular; CVOT, cardiovascular outcomes trial; DKA, diabetic ketoacidosis; DKD, diabetic kidney disease; DPP-4, dipeptidyl peptidase 4; eGFR, estimated glomerular filtration rate; FDA, U.S. Food and Drug Administration; GI, gastrointestinal; GIP, gastric inhibitory polypeptide; GLP-1 RA, glucagon-like peptide 1 receptor agonist; HF, heart failure; NASH, nonalcoholic steatohepatitis; MACE, major adverse cardiovascular events; SGLT2, sodium–glucose cotransporter 2; SQ, subcutaneous; T2DM, type 2 diabetes mellitus.
For agent-specific dosing recommendations, please refer to manufacturers’ prescribing information.
Tsapas et al. (62).