Abstract
The outer membrane protein ChuA responsible for hemin utilization has been recently identified in several pathogenic Escherichia coli strains. We report that the regulatory protein RfaH influences ChuA expression in the uropathogenic E. coli strain 536. In an rfaH mutant, the chuA transcript as well as the ChuA protein levels were significantly decreased in comparison with those in the wild-type strain. Within the chuA gene, a consensus motif known as the JUMPStart (just upstream of many polysaccharide associated gene starts) sequence was found, which is shared by RfaH-affected operons. Furthermore, the presence of two different subclasses of the chuA determinant and their distribution in E. coli pathogroups are described.
The availability of iron, an essential nutrient for bacterial growth, is severely limited in mammalian hosts. In order to compete with the host for iron, pathogenic bacteria have developed different mechanisms to acquire this essential growth factor (10). Low-molecular-weight chelators (siderophores) are secreted by several pathogens. These molecules liberate Fe3+ from host carriers and transport it into bacterial cells. Alternatively, many pathogenic bacteria can directly utilize iron-containing host compounds through specific receptors. Several gram-negative pathogens, e.g., Haemophilus influenzae type b (6), yersiniae (34, 37), Vibrio cholerae (26), neisseriae (17, 35), and Shigella dysenteriae (21), express outer membrane proteins involved in the utilization of heme and its protein complexes as iron sources. In Escherichia coli O157:H7 the gene chuA, which codes for a 69-kDa outer membrane protein responsible for heme uptake, was recently identified (38). The chuA nucleotide sequence shows high homology to that of the formerly described shuA gene of S. dysenteriae type 1 (40). The gene is part of a larger locus, termed the heme transport locus, which appears to be widely distributed among pathogenic E. coli strains (41). This locus contains eight open reading frames and is located at 78.7 min of the E. coli K-12 chromosome.
The ability to use heme and/or hemoglobin might be especially advantageous to pathogenic bacteria. These pathogens often secrete cytotoxins, which gain access to the intracellular heme reservoir besides initiating tissue invasion. Cytotoxin production coupled with the capability to utilize heme and/or hemoglobin could serve as an effective iron acquisition strategy during the progression of infection.
RfaH regulates the transcription of long operons probably at the level of transcription antitermination, hence suppressing operon polarity (2, 18). These operons share a conserved motif, which was identified for the first time in polysaccharide-associated operons and was therefore termed the JUMPStart (for just upstream of many polysaccharide-associated gene starts) sequence (12). The most-conserved part of this 39-bp motif is an 8-bp sequence termed the ops element (for operon polarity suppressor), which is always associated with a direct repeat that shows less similarity to the standard element (2). Deletion of the ops element and/or other parts of the JUMPStart sequence results in transcriptional polarity of the affected operons (19, 24). A similar transcriptional pattern is observed in rfaH mutants, suggesting that the regulation of these operons by RfaH is dependent on the presence of the JUMPStart motif. In this study we investigated the effect of RfaH on the expression of the E. coli hemin receptor protein ChuA.
Bacterial strains and culture conditions.
The uropathogenic E. coli strain 536 was isolated from a patient with acute pyelonephritis (3). In the mutant strain 536rfaH::cat, the rfaH gene was inactivated by insertion of a chloramphenicol acetyltransferase (cat) cassette. The insertion was performed by allelic exchange as previously described (23). trans-complementation of rfaH was achieved by supplying the mutant strain with the plasmid pSMK1, which carried rfaH together with its promoter region cloned into the vector pGEM-T Easy (Promega). The strains used in Southern hybridization experiments are listed in Table 1. The enterohemorrhagic E. coli (EHEC) strain 95004730 and the enteroaggregative E. coli (EAggEC) strain DPA065 were provided by Robert Pringle (Victorian Infectious Diseases Reference Laboratory, North Melbourne, Australia) and Anna Giammanco (Dipartimento di Igiene e Microbiologia, University of Palermo, Palermo, Italy), respectively. The origins of all other E. coli wild-type strains are referenced in Table 1. Bacteria were grown routinely in Luria-Bertani broth or Luria-Bertani broth solidified with 1.5% agar (Difco, Detroit, Mich.). In iron-restricted studies, a 0.4 mM concentration of the iron chelator 2,2′-dipyridyl (Sigma, Deisenhofen, Germany) was added to the media. When appropriate, the medium was supplemented with the following antibiotics at the indicated concentrations: ampicillin, 100 μg/ml; chloramphenicol, 30 μg/ml.
TABLE 1.
Occurrence of chuA and the two distinct chuA upstream regions among pathogenic E. coli strains
| Strain | Serotype | Pathogroupa | Hybridization withb:
|
Reference or source | ||
|---|---|---|---|---|---|---|
| Probe-1 (chuA)c | Probe-2 | Probe-3 | ||||
| HB101 | K-12 | − | − | − | 5 | |
| 536 | O6:K15:H31 | UPEC | 12 | + | − | 3 |
| J96 | O4:K6 | UPEC | 12 | + | − | 13 |
| AD110 | O6:K2 | UPEC | 12 | + | − | 39 |
| 764 | O18:K5:H5/11 | UPEC | 12 | + | − | 27 |
| 2980 | O18:K5:H5/11 | UPEC | 12 | + | − | 27 |
| RZ439 | O6:K5 | UPEC | 12 | + | − | 43 |
| RZ441 | O6:K5 | UPEC | 12 | + | − | 43 |
| RS218 | O18:K1:H7 | NBM | 12 | + | − | 33 |
| IHE3034 | O18:K1:H7/9 | NBM | 12 | + | − | 15 |
| IHE3036 | O18:K1:H7/9 | NBM | 12 | + | − | 15 |
| EDL933 | O157:H7 | EHEC | 11 | − | + | 25 |
| 9167/91 | O157:H7 | EHEC | 11 | − | + | 7 |
| 5159/91 | O157:H7 | EHEC | 11 | − | + | 31 |
| 86-24 | O157:H7 | EHEC | 11 | − | + | 8 |
| E32511 | O157:H7 | EHEC | 11 | − | + | 11 |
| 6578/93 | O157:H7 | EHEC | 11 | − | + | 31 |
| SF493/89 | O157:H− | EHEC | 11 | − | + | 14 |
| 3574/92 | O157:H− | EHEC | 11 | − | + | 7 |
| 3978/91 | O157:H− | EHEC | 11 | − | + | 30 |
| 5291/92 | O157:H− | EHEC | 11 | − | + | 30 |
| 2907/97 | O55:H6 | EHEC | 11 | − | + | This study |
| 5720/96 | O26:H− | EHEC | − | − | − | 42 |
| 3697/97 | O26:H− | EHEC | − | − | − | 32 |
| ED147 | O26:H11 | EHEC | − | − | − | 29 |
| 5714/96 | O103:H2 | EHEC | − | − | − | 32 |
| ED142 | O111:H− | EHEC | − | − | − | 29 |
| 78/92 | O111:H− | EHEC | − | − | − | 36 |
| 95004730 | O111:H− | EHEC | − | − | − | RPe |
| E2348/69 | O127:H6 | EPEC | 12 | + | − | 20 |
| 179/2 | O55:H6 | EPEC | 12 | + | − | This study |
| 156A | O55:H7 | EPEC | − | − | − | 4 |
| 182A | O55:H7 | EPEC | 11 | − | + | 4 |
| 37-4 | O55:H− | EPEC | − | − | − | 20 |
| 76-5 | O143:HND | EIEC | 11 | − | + | This study |
| 12860 | O124:HND | EIEC | − | − | − | This study |
| EDL1284 | NDd | EIEC | 11 | − | + | This study |
| C9221a | O6:K15:H16 | ETEC | − | − | − | This study |
| DPA065 | O119:HND | EAggEC | − | − | − | AGf |
| 5477/94 | O86:H7 | EAggEC | − | − | − | This study |
| 7484/94 | O86:H18 | EAggEC | − | − | − | This study |
| DDC4441 | O128:HND | EAggEC | − | − | − | This study |
| 17-2 | O3:H2 | EAggEC | − | − | − | 40 |
| 5464/95 | O3:H2 | EAggEC | − | − | − | This study |
Abbreviations: UPEC, uropathogenic E. coli; NBM, newborn meningitis-causing E. coli; ETEC, enterotoxigenic E. coli.
The probes used are described in Fig. 3.
The numbers indicate the size of the fragments hybridized with the probe (in kilobases) after digestion of chromosomal DNA with BglI.
ND, not determined.
RP, Robert Pringle (Victoria Infectious Diseases Reference Laboratory).
AG, Anna Giammanco (Dipartimento di Igiene e Microbiologia, University of Palermo).
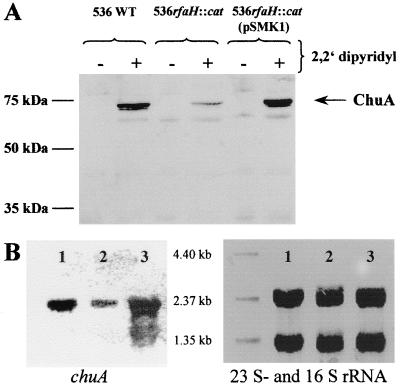
Expression of ChuA is decreased in the rfaH mutant of strain 536.
The ChuA protein levels expressed in E. coli 536 and its derivatives were determined by Western blotting (Fig. 1A). Whole-cell extracts obtained from bacteria grown in normal and iron-restricted media were separated on a 10% polyacrylamide gel and were blotted onto a nitrocellulose membrane. The blocked membranes were treated with an anti-HemR antiserum (kindly provided by J. Heesemann) and were developed as described elsewhere (28). HemR is the hemin receptor protein of Yersinia enterocolitica. The HemR antiserum was proven to be cross-reactive with ChuA of E. coli 536 (data not shown). The quantity of ChuA protein was strongly reduced in the rfaH-negative strain compared to the wild type. trans-complementation of the mutant strain with rfaH (on pSMK1) restored higher levels of ChuA. No ChuA protein was detectable in the absence of 2,2′-dipyridyl, indicating that expression of ChuA is dependent on the availability of free iron.
FIG. 1.
Influence of RfaH on chuA expression of E. coli strain 536. (A) Detection of ChuA levels by Western blot analysis of whole-cell extracts of E. coli strain 536 and its derivatives using a HemR-specific antiserum. The strains were grown in the presence (+) or absence (−) of 0.4 mM 2,2′-dipyridyl. (B) Analysis of chuA transcript levels of E. coli strain 536 and its derivatives. An enhanced chemiluminescence-labeled chuA-specific probe was hybridized to total RNA isolated from strain 536 (lane 1), 536rfaH::cat (lane 2), 536rfaH::cat (pSMK1) (lane 3). The 23S and 16S rRNA were stained with 0.3% methylene blue after transfer of separated total RNA to a nylon membrane (internal control).
To investigate whether the altered expression of ChuA protein was a consequence of decreased chuA transcription in the rfaH mutant, we performed Northern blot analysis (Fig. 1B). Total RNA was isolated from bacteria harvested from iron-restricted medium using the RNeasy Mini Kit (Qiagen, Hilden, Germany). Northern blot analysis was performed as described previously (1). Ten micrograms of isolated RNA per lane was separated on a 1.2% agarose-formaldehyde gel and was transferred overnight to a nylon membrane (Biodyne B; Pall Ltd., Portsmouth, England) by capillary blotting. The DNA probe specific for the 3′ end of chuA was generated by PCR using the primers 5′-GTCGCTTCTATACCAACTATTGGGTG-3′ and 5′-CCGTTACGACCATCCTGTG-3′ and was labeled with the ECL direct labeling system (Amersham-Pharmacia, Freiburg, Germany). Hybridization was carried out overnight at 42°C as described by Amersham-Pharmacia. Before luminography, the membrane was washed twice for 15 min in 0.5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.4% sodium dodecyl sulfate (SDS) (50°C) and then twice for 5 min in 2× SSC (20°C). The chuA-specific DNA probe hybridized to a 2.2 to 2.3-kb mRNA, which corresponds to the chuA transcript (Fig. 1B). The absence of an intact rfaH gene resulted in reduced levels of chuA mRNA; however, the length of the transcript was not altered. Overexpression of RfaH (536rfaH::cat carrying pSMK1) manifested in an increased chuA transcription compared to the level found in the wild-type strain. RfaH has been known as a regulator which influences the transcription of long operons encoding cell surface and extracellular components that are important for bacterial fertility and virulence. These include the hly, rfa, rfb, tra, cps, and kps operons that direct the synthesis of α-hemolysin, lipopolysaccharide core, O antigen, F factor, and group I and group II capsule, respectively (2). The hemin receptor ChuA is also anchored in the outer membrane of pathogenic E. coli strains and is considered to be a potential virulence factor. As the encoding gene (chuA) is transcribed as a monocistronic mRNA, the way RfaH is involved in transcriptional regulation of chuA transcription seems to be inconsistent with the present view that RfaH acts as a transcriptional antiterminator without affecting transcription initiation (18).
Coregulation of different determinants involved in pathogenicity is energetically advantageous for pathogenic bacteria. This is especially true for components of a complex system which are functionally related. α-Hemolysin expression and hemin uptake are both regulated by iron (16, 21), suggesting that the utilization of heme compounds liberated from eukaryotic cells is an important iron acquisition strategy during infection. Coupled regulation by RfaH gives further evidence that the function of the E. coli hemin uptake system (chu) is dependent on secreted hemolysin.
Sequence analysis of the chuA gene of E. coli strain 536.
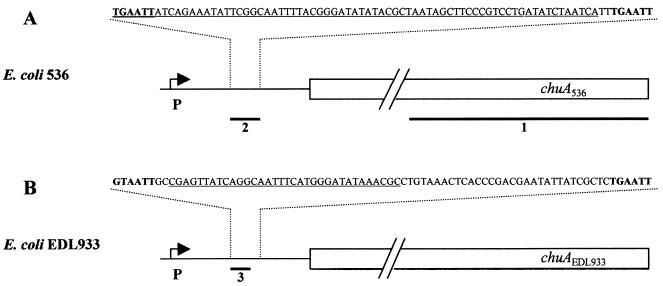
Sequencing of the chuA gene was performed from a cosmid clone of E. coli 536 using an ABI Prism 310 automatic sequencer. It was previously shown that RfaH-regulated operons carry a conserved region known as the JUMPStart sequence (2). Within the chuA gene of E. coli strain 536, a similar motif was identified. A comparison of this motif to JUMPStart sequences of other E. coli operons known to be regulated by RfaH is shown in Fig. 2. The 39-bp region found in the chuA gene is located 1,158 bp downstream of the start codon. It contains an ops-like motif with an additional conserved C base located downstream of the ops element. In the 5′ region of the JUMPStart sequence, a relatively well-conserved direct repeat could be identified with relevant spacing similar to those of other JUMPStart sequences.
FIG. 2.
Comparison of JUMPStart sequences from different E. coli operons. Boldface letters denote the ops element; underlined bases represent the imperfect repeats within the JUMPStart sequences. Accession numbers or references for the following sequences are as follows: cps, AF104912; kps, X53819; rfb, U09876; tra, U01159; rfa, M86935; p152 hly, M14107 and X07565; 2001 hly, reference 24; J96 hly, M10133; 536 hlyI and hlyII, G. Nagy and G. Blum-Oehler, unpublished data; 536 chu, AF280396; EDL933 chu, U67920.
The 1,983-bp coding region of chuA shows high homology to the corresponding sequences derived from E. coli O157:H7 (38) and S. dysenteriae (22). The potential promoter region is located about 300 bp upstream of the start codon, and is overlapped by a putative Fur box. The presence of this motif neighboring the promoter explains the observed effect of iron availability on ChuA protein levels. In contrast to the high homology between the coding regions of different chuA and shuA determinants, the E. coli 536-specific chuA upstream region showed less similarity to the corresponding regions of E. coli O157:H7 and S. dysenteriae. A 74-bp region located between the putative promoter and the start codon of the E. coli 536-specific determinant is replaced by a totally different 73-bp motif in S. dysenteriae and E. coli O157:H7 (Fig. 3). In the uropathogenic strain, this region is flanked by 6-bp direct repeats that might have served as a site for recombination. In E. coli O157:H7, this region is bordered by similar, nevertheless imperfect, repeats.
FIG. 3.
Genetic map of the uropathogenic E. coli strain 536-specific chuA gene (A) and that of EHEC strain EDL933 (B). The chuA coding regions are indicated by boxes; the 5′ flanking regions are indicated by single lines. The arrows labeled with P denote the promoters. The sequences of the upstream element specific for strain 536 or strain EDL933 are given. Bases in boldface type represent the direct repeats flanking dissimilar regions. The numbers and thick lines denote the probes used for Southern hybridization (see text). The sequences of oligonucleotides used as probes are underlined.
To investigate the distribution of the two different identified 5′-flanking sequences of chuA, several E. coli strains representing different pathogroups were tested by Southern hybridization. Chromosomal DNA was isolated as described before (9). The DNA was digested with BglI prior to separation on a 0.8% agarose gel and subsequent transfer to a nylon membrane (Biodyne B; Pall Ltd.) The presence of chuA in the genomes was proven by hybridization of a 600-bp probe derived from the well-conserved 3′ part of chuA (probe-1). Generation, labeling, and detection of the chuA-specific probe as well as the hybridization procedure were performed as described above for Northern blot analysis. Oligonucleotides derived from the dissimilar upstream regions were used to analyze the distribution of the different 5′ flanking regions. Probe-2 (derived from strain 536 [Fig. 3]) (5′-TGA ATT ATC AGA AAT ATT CGG CAA TTT TAC GGG ATA TAT ACG CTA ATA GCT TCC CGT GGT GAT ATC TAA TCA-3′) and probe-3 (derived from the strain EDL933 [Fig. 3]) (5′-CGA GTT ATC AGG CAA TTT CAT GGG ATA TAA ACG C-3′) were purchased from ARK Scientific GmbH (Darmstadt, Germany). The probes were labeled with digoxigenin using the DIG Oligonucleotide 3′-End Labeling kit (Roche, Mannheim, Germany). Prehybridization and hybridization were carried out in high-SDS hybridization buffer at 30°C for 4 h and overnight, respectively. The filters were washed twice for 10 min at room temperature in 2× SSC–0.1% SDS. Hybridized oligonucleotides were detected using the DIG Luminescent Detection kit (Roche) following the standard protocol provided by the manufacturer. The results of the hybridization experiments are summarized in Table 1.
The probe specific for the 3′ end of the chuA gene (probe-1) hybridized with numerous intestinal and all extraintestinal pathogenic E. coli strains. However, the chuA-specific probe hybridized to two distinct bands: to a larger DNA fragment (∼12 kb) in case of the extraintestinal and some of the enteropathogenic E. coli (EPEC) strains, whereas in EHEC O157, enteroinvasive E. coli (EIEC) and some other EPEC strains the chuA probe hybridized to a smaller fragment (∼11 kb). In correspondence with former investigations, none of the tested non-O157-EHEC, EAggEC, and enterotoxigenic E. coli representatives carried chuA (41). The oligonucleotide specific for the chuA upstream region of strain 536 (probe-2) hybridized to all strains that carried chuA on the 12-kb fragment, while that which originated from the O157:H7 strain EDL933 (probe-3) hybridized to the 11-kb fragment, suggesting that two distinct variants of the chuA determinant, which show differences in their flanking sequences, exist. The existence of these two variants and their patterned distribution among different pathogroups provides further evidence for the clonality of E. coli pathogens. Whether the differences in the chuA upstream regions have any influence on the regulation of chuA expression still needs to be clarified.
Nucleotide sequence accession number.
The nucleotide sequence of the E. coli strain 536-specific chuA gene has been deposited in the GenBank database (accession number AF280396).
Acknowledgments
We thank Jürgen Heesemann for supplying the HemR antiserum.
Our work was supported by the Deutsche Forschungsgemeinschaft (Ha 1434/8-2, SFB 479) and the Fonds der Chemischen Industrie. G.N. was supported by a grant from the Bayerische Forschungsstiftung.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 4. New York, N.Y: John Wiley & Sons; 1987. [Google Scholar]
- 2.Bailey M J A, Hughes C, Koronakis V. RfaH and the ops element, components of a novel system controlling bacterial transcription elongation. Mol Microbiol. 1997;26:845–851. doi: 10.1046/j.1365-2958.1997.6432014.x. [DOI] [PubMed] [Google Scholar]
- 3.Berger H, Hacker J, Juarez A, Hughes C, Goebel W. Cloning of the chromosomal determinants encoding hemolysin production and mannose-resistant hemagglutination in Escherichia coli. J Bacteriol. 1982;152:1241–1247. doi: 10.1128/jb.152.3.1241-1247.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bokete T N, Whittam T S, Wilson R A, Clausen C R, O'Callahan C M, Moseley S L, Fritsche T R, Tarr P I. Genetic and phenotypic analysis of Escherichia coli with enteropathogenic characteristics isolated from Seattle children. J Infect Dis. 1997;175:1382–1389. doi: 10.1086/516470. [DOI] [PubMed] [Google Scholar]
- 5.Boyer H V, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 6.Cope L D, Yogev R, Muller-Eberhard U, Hansen E J. A gene cluster involved in the utilization of both free heme and heme:hemopexin by Haemophilus influenzae type b. J Bacteriol. 1995;177:2644–2653. doi: 10.1128/jb.177.10.2644-2653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datz M, Janetzki-Mittmann C, Franke S, Gunzer F, Schmidt H, Karch H. Analysis of the enterohemorrhagic Escherichia coli O157 DNA region containing lambdoid phage gene p and Shiga-like toxin structural genes. Appl Environ Microbiol. 1996;62:791–797. doi: 10.1128/aem.62.3.791-797.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnenberg M S, Tzipori S, McKee M L, O'Brien A D, Alroy J, Kaper J B. The role of the eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J Clin Investig. 1993;3:1418–1424. doi: 10.1172/JCI116718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimberg J, Maguire S, Belluscio L. A simple method for the preparation of plasmid and chromosomal DNA. Nucleic Acids Res. 1989;21:8893. doi: 10.1093/nar/17.21.8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerinot M L. Microbial iron transport. Annu Rev Microbiol. 1994;48:743–772. doi: 10.1146/annurev.mi.48.100194.003523. [DOI] [PubMed] [Google Scholar]
- 11.Hii J H, Gyles C, Morooka T, Karmali M A, Clarke R, De Grandis S, Brunton J L. Development of verotoxin 2- and verotoxin 2 variant (VT2v)-specific oligonucleotide probes on the basis of the nucleotide sequence of the B cistron of VT2v from Escherichia coli E32511 and B2F1. J Clin Microbiol. 1991;29:2704–2709. doi: 10.1128/jcm.29.12.2704-2709.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hobbs M, Reeves P R. The JUMPstart sequence: a 39 bp element common to several polysaccharide gene clusters. Mol Microbiol. 1994;12:855–856. doi: 10.1111/j.1365-2958.1994.tb01071.x. [DOI] [PubMed] [Google Scholar]
- 13.Hull R A, Gill R E, Hsu P, Minshew B H, Falkow S. Construction and expression of recombinant plasmids encoding type 1 or d-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981;33:933–936. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karch H, Böhm H, Schmidt H, Gunzer F, Aleksic S, Heesemann J. Clonal structure and pathogenicity of Shiga-like toxin-producing, sorbitol-fermenting Escherichia coli O157:H−. J Clin Microbiol. 1993;31:1200–1205. doi: 10.1128/jcm.31.5.1200-1205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korhonen T K, Valtonen M V, Parkkinen J, Vaisänen-Rhen V, Finne J, Ørskov F, Ørskov I, Svenson S B, Mäkelä P H. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect Immun. 1985;48:486–491. doi: 10.1128/iai.48.2.486-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebek G, Grünig H M. Relation between the hemolytic property and iron metabolism in Escherichia coli. Infect Immun. 1985;50:682–686. doi: 10.1128/iai.50.3.682-686.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee B C. Isolation of haemin-binding proteins of Neisseria gonorrhoeae. J Med Microbiol. 1992;36:121–127. doi: 10.1099/00222615-36-2-121. [DOI] [PubMed] [Google Scholar]
- 18.Leeds J A, Welch R A. RfaH enhances elongation of Escherichia coli hlyCABD mRNA. J Bacteriol. 1996;178:1850–1857. doi: 10.1128/jb.178.7.1850-1857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leeds J A, Welch R A. Enhancing transcription through the Escherichia coli hemolysin operon, hlyCABD: RfaH and upstream JUMPstart DNA sequences function together via a postinitiation mechanism. J Bacteriol. 1997;179:3519–3527. doi: 10.1128/jb.179.11.3519-3527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine M M, Bergquist E J, Nalin D R, Waterman D H, Hornick R B, Young C R, Sotman S. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet. 1978;i:1119–1122. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- 21.Mills M, Payne S M. Genetics and regulation of heme iron transport in Shigella dysenteriae and detection of an analogous system in Escherichia coli O157:H7. J Bacteriol. 1995;177:3004–3009. doi: 10.1128/jb.177.11.3004-3009.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mills M, Payne S M. Identification of shuA, the gene encoding the heme receptor of Shigella dysenteriae, and analysis of invasion and intracellular multiplication of a shuA mutant. Infect Immun. 1997;65:5358–5363. doi: 10.1128/iai.65.12.5358-5363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mobley H L, Jarvis K G, Elwood J P, Whittle D I, Lockatell C V, Russell R G, Johnson D E, Donnenberg M S, Warren J W. Isogenic P-fimbrial deletion mutants of pyelonephritogenic Escherichia coli: the role of alpha Gal(1-4) beta Gal binding in virulence of a wild-type strain. Mol Microbiol. 1993;10:143–155. doi: 10.1111/j.1365-2958.1993.tb00911.x. [DOI] [PubMed] [Google Scholar]
- 24.Nieto J M, Bailey M J A, Hughes C, Koronakis V. Suppression of transcription polarity in the Escherichia coli haemolysin operon by a short upstream element shared by polysaccharide and DNA transfer determinants. Mol Microbiol. 1996;19:705–713. doi: 10.1046/j.1365-2958.1996.446951.x. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien A D, Lively T A, Chang T W, Gorbach S L. Purification of Shigella dysenteriae 1 (Shiga)-like toxin from Escherichia coli O157:H7 strain associated with haemorrhagic colitis. Lancet. 1983;ii:573. doi: 10.1016/s0140-6736(83)90601-3. [DOI] [PubMed] [Google Scholar]
- 26.O'Malley S M, Mouton S L, Occhino D A, Deanda M T, Rashidi J R, Fuson K L, Rashidi C E, Mora M Y, Payne S M, Henderson D P. Comparison of the heme iron utilization systems of pathogenic vibrios. J Bacteriol. 1999;181:3594–3598. doi: 10.1128/jb.181.11.3594-3598.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ott M, Hacker J, Schmoll T, Jarchau T, Korhonen T K, Goebel W. Analysis of the genetic determinants coding for the S-fimbrial adhesin (sfa) in different Escherichia coli strains causing meningitis or urinary tract infections. Infect Immun. 1986;54:646–653. doi: 10.1128/iai.54.3.646-653.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ritter A, Gally D L, Olsen P B, Dobrindt U, Friedrich A, Klemm P, Hacker J. The Pai-associated leuX specific tRNA5Leu affects type 1 fimbriation in pathogenic Escherichia coli by control of FimB recombinase expression. Mol Microbiol. 1997;25:871–882. doi: 10.1111/j.1365-2958.1997.mmi517.x. [DOI] [PubMed] [Google Scholar]
- 29.Rüssmann H, Kothe E, Schmidt H, Franke S, Harmsen D, Caprioli A, Karch H. Genotyping of Shiga-like toxin genes in non-O157 Escherichia coli strains associated with haemolytic uraemic syndrome. J Med Microbiol. 1995;42:404–410. doi: 10.1099/00222615-42-6-404. [DOI] [PubMed] [Google Scholar]
- 30.Rüssmann H, Schmidt H, Caprioli A, Karch H. Highly conserved B-subunit genes of Shiga-like toxin II variants found in Escherichia coli O157 strains. FEMS Microbiol Lett. 1994;118:335–340. doi: 10.1111/j.1574-6968.1994.tb06849.x. [DOI] [PubMed] [Google Scholar]
- 31.Rüssmann H, Schmidt H, Heesemann J, Caprioli A, Karch H. Variants of Shiga-like toxin II constitute a major toxin component in Escherichia coli O157 strains from patients with haemolytic uraemic syndrome. J Med Microbiol. 1994;40:338–343. doi: 10.1099/00222615-40-5-338. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt H, Geitz C, Tarr P I, Frosch M, Karch H. Non-O157:H7 pathogenic Shiga toxin-producing Escherichia coli: phenotypic and genetic profiling of virulence traits and evidence for clonality. J Infect Dis. 1999;179:115–123. doi: 10.1086/314537. [DOI] [PubMed] [Google Scholar]
- 33.Silver R P, Finn C W, Vann W F, Aaronson W, Schneerson R, Kretschmer P J, Garon C F. Molecular cloning of the K1 capsular polysaccharide genes of E. coli. Nature. 1981;289:696–698. doi: 10.1038/289696b0. [DOI] [PubMed] [Google Scholar]
- 34.Stojiljkovic I, Hantke K. Hemin uptake system of Yersinia enterocolitica: similarities with other TonB-dependent systems in gram-negative bacteria. EMBO J. 1992;11:4359–4367. doi: 10.1002/j.1460-2075.1992.tb05535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stojiljkovic I, Hwa V, de Saint Martin L, O'Gaora P, Nassif X, Heffron F, So M. The Neisseria meningitidis haemoglobin receptor: its role in iron utilization and virulence. Mol Microbiol. 1995;15:531–541. doi: 10.1111/j.1365-2958.1995.tb02266.x. [DOI] [PubMed] [Google Scholar]
- 36.Tarr P I, Neill M A, Clausen C R, Newland J W, Neill R J, Moseley S L. Genotypic variation in pathogenic Escherichia coli O157:H7 isolated from patients in Washington, 1984-1987. J Infect Dis. 1989;159:344–347. doi: 10.1093/infdis/159.2.344. [DOI] [PubMed] [Google Scholar]
- 37.Thompson J M, Jones H A, Perry R D. Molecular characterization of the hemin uptake locus (hmu) from Yersinia pestis and analysis of hmu mutants for hemin and hemoprotein utilization. Infect Immun. 1999;67:3879–3892. doi: 10.1128/iai.67.8.3879-3892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torres A G, Payne S M. Haem iron-transport system in enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol. 1997;23:825–833. doi: 10.1046/j.1365-2958.1997.2641628.x. [DOI] [PubMed] [Google Scholar]
- 39.van Die I, van Geffen B, Hoekstra W, Bergmans H. Type 1C fimbriae of a uropathogenic Escherichia coli strain: cloning and characterization of the genes involved in the expression of the 1C antigen and nucleotide sequence of the subunit gene. Gene. 1985;34:187–196. doi: 10.1016/0378-1119(85)90127-1. [DOI] [PubMed] [Google Scholar]
- 40.Vial P A, Robins-Browne R, Lior H, Prado V, Kaper J B, Nataro J P, Maneval D, Elsayed A, Levine M M. Characterization of enteroadherent-aggregative Escherichia coli, a putative agent of diarrheal disease. J Infect Dis. 1988;158:70–79. doi: 10.1093/infdis/158.1.70. [DOI] [PubMed] [Google Scholar]
- 41.Wyckoff E E, Duncan D, Torres A G, Mills M, Maase K, Payne S M. Structure of the Shigella dysenteriae haem transport locus and its phylogenetic distribution in enteric bacteria. Mol Microbiol. 1998;28:1139–1152. doi: 10.1046/j.1365-2958.1998.00873.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W-L, Bielaszewska M, Liesegang A, Tschäpe H, Schmidt H, Bitzan M, Karch H. Molecular characteristics and epidemiological significance of Shiga toxin-producing Escherichia coli O26 strains. J Clin Microbiol. 2000;38:2134–2140. doi: 10.1128/jcm.38.6.2134-2140.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zingler G, Blum G, Falkenhagen U, O/rskov I, O/rskov F, Hacker J, Ott M. Clonal differentiation of uropathogenic Escherichia coli isolates of serotype O6:K5 by fimbrial antigen typing and DNA long-range mapping techniques. Med Microbiol Immunol. 1993;182:13–24. doi: 10.1007/BF00195947. [DOI] [PubMed] [Google Scholar]