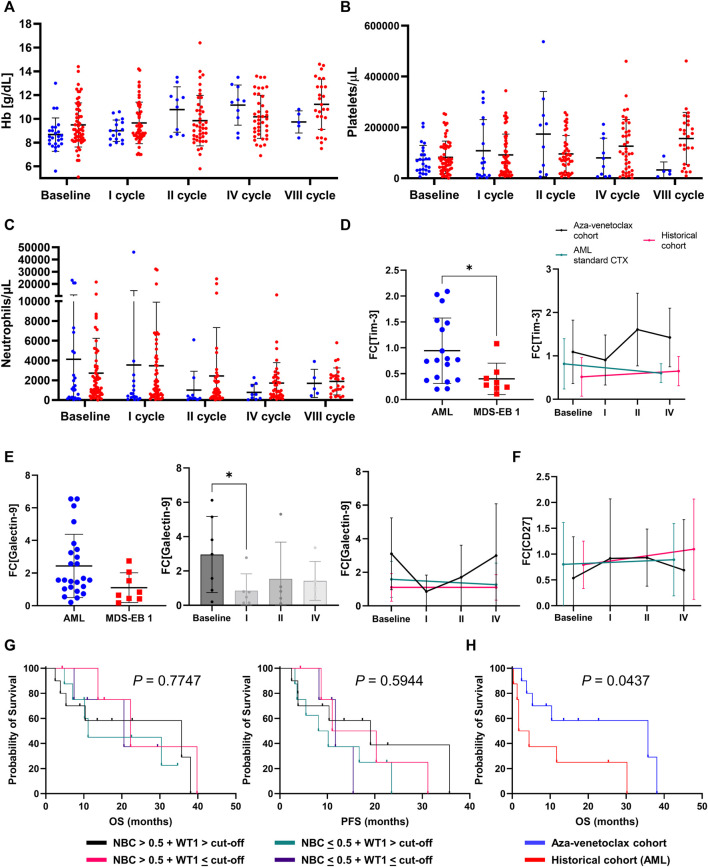
FIGURE 3.
Laboratory parameters and prognosticators (A) hemoglobin (Hb) levels (B) platelets count, and (C) absolute neutrophil count variations at baseline and during treatment in azacytidine plus venetoclax (aza-venetoclax cohort) or to azacytidine as single agent (historical cohort) groups are reported (D) differences in Tim-3 expression [reported as fold-change (FC) to healthy controls] based on disease [acute myeloid leukemia (AML) and high-risk myelodysplastic syndromes (MDS) vs. MDS with excess of blast of type 1 (MDS-EB 1)] or treatment type [azacytidine + venetoclax vs. azacytidine alone vs. standard chemotherapy (CTX)]. Similarly (E) galectin-9 and (F) CD27 FC variations are displayed based on disease type and during treatment (G) Overall survival (OS) and progression-free survival (PFS) are reported for patients treated with azacytidine plus venetoclax divided by normalized WT1 expression levels and flow cytometric normalized blast count (NBC) values (H) OS of patients with WT1 > cut-off (50 copies in peripheral blood or 250 copies in bone marrow) + NBC >0.5 were compared between aza-venetoclax and historical AML cohort.