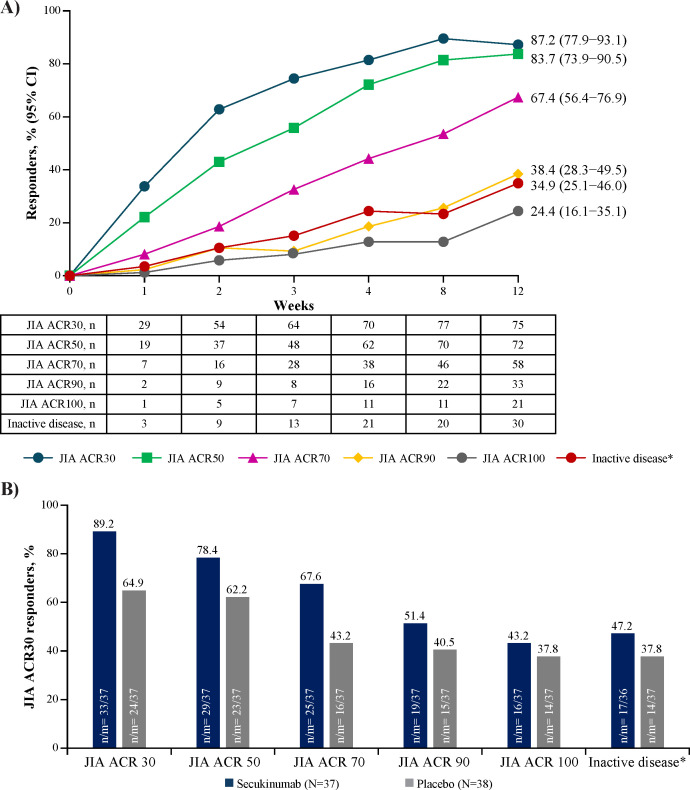
Figure 2.
Improvement in ACR responses from baseline with open-label secukinumab in TP1 (A), and improvement from baseline at the end of TP2 (B). *Inactive disease status. 95% CI are from NRI analysis of ACR response derived relative to baseline. N=86 N, number of patients in the full analysis set; n, number of patients with response (B). *Inactive disease status. NRI analysis. ACR, American College of Rheumatology; JIA, juvenile idiopathic arthritis; m, total number of patients with an assessment at the end of TP2; N, total number of patients in the treatment group; n, number of patients who satisfy the criteria; NRI, non-responder imputation; TP, treatment period.