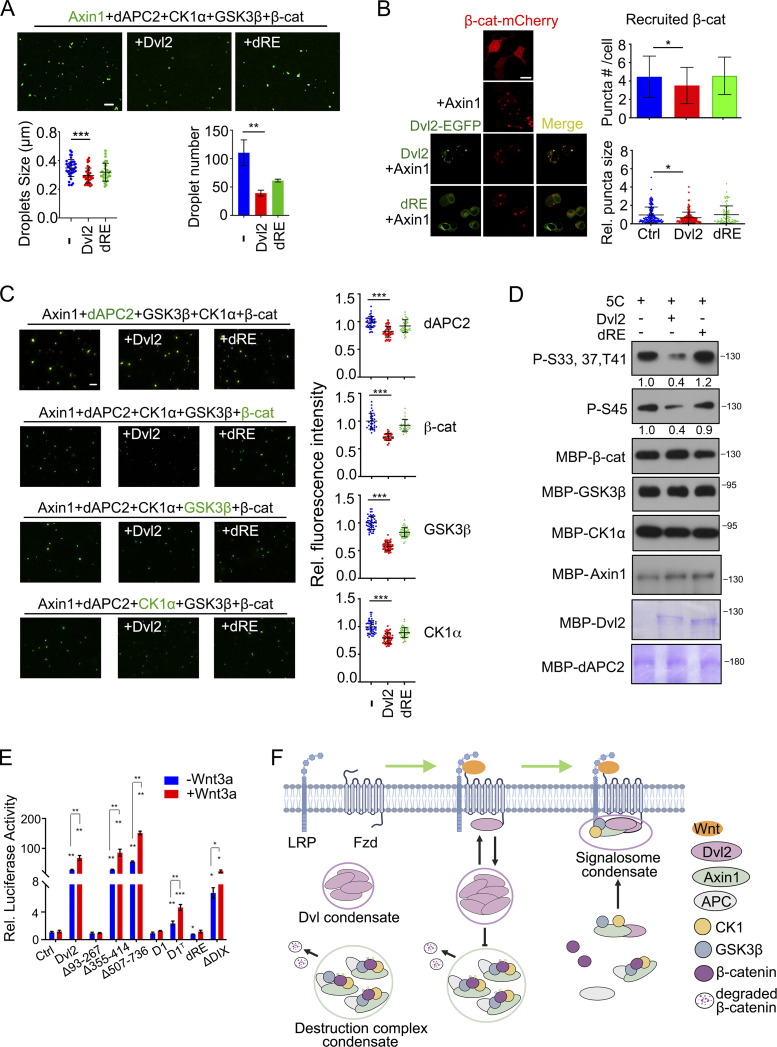
Figure 4.
Dvl2 LLPS disrupts the organization and function of the β-catenin destruction complex. (A) Alexa Fluor 488 NHS ester labeled 1 µM Axin1 protein (green) and other destruction complex components (1 µM) were mixed with or without Dvl2 or the dRE deletion and subjected to in vitro LLPS assay. The number and size of droplets were counted from three independent fields. (B) Confocal images of β-catenin-mCherry puncta when Axin1 was expressed with or without Dvl2-EGFP or Dvl2(dRE)-EGFP in Dvl1/2/3 KO cells. The number and size of puncta were counted from three independent cells. The puncta size was normalized to that in the control group. (C) Destruction complex components were mixed with or without Dvl2 and the Dvl2(dRE) for in vitro LLPS assay. In each assay, only one protein was labeled with Alexa Fluor 488 NHS ester. Fluorescence images (left) and the fluorescence intensity of the labeled protein recruited into the Axin1 droplets (right) are shown. 5C: Axin1, dAPC, β-catenin, CK1α, and GSK3β. (D) Dvl2 protein or the Dvl2(dRE) mutant (1 µM) were incubated with 1 µM Axin1, 50 nM GSK3β and 2.5 µM CK1α for in vitro phosphorylation. The band intensity of phosphorylated β-catenin was normalized to total β-catenin protein. 5C: Axin1, dAPC, β-catenin, CK1α and GSK3β. (E) Dvl1/2/3 KO cells were transfected with TopFlash-luciferase reporter and WT Dvl2-EGFP or its mutants, then treated with or without Wnt3a conditional medium for 12 h and harvested for determination of luciferase activity. The expression level of Dvl2 WT and mutant proteins in the reporter assay was detected with immunoblotting (bottom panel). (F) Working model of Dvl2 LLPS in assembly of the Wnt receptor signalosome and disruption of the β-catenin destruction complex. Statistical analyses were performed with the two-tailed unpaired t test. Data are shown in A, B, C, and E as mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Scale bars in B, 10 µm; A and C, 2 µm. Source data are available for this figure: SourceData F4.