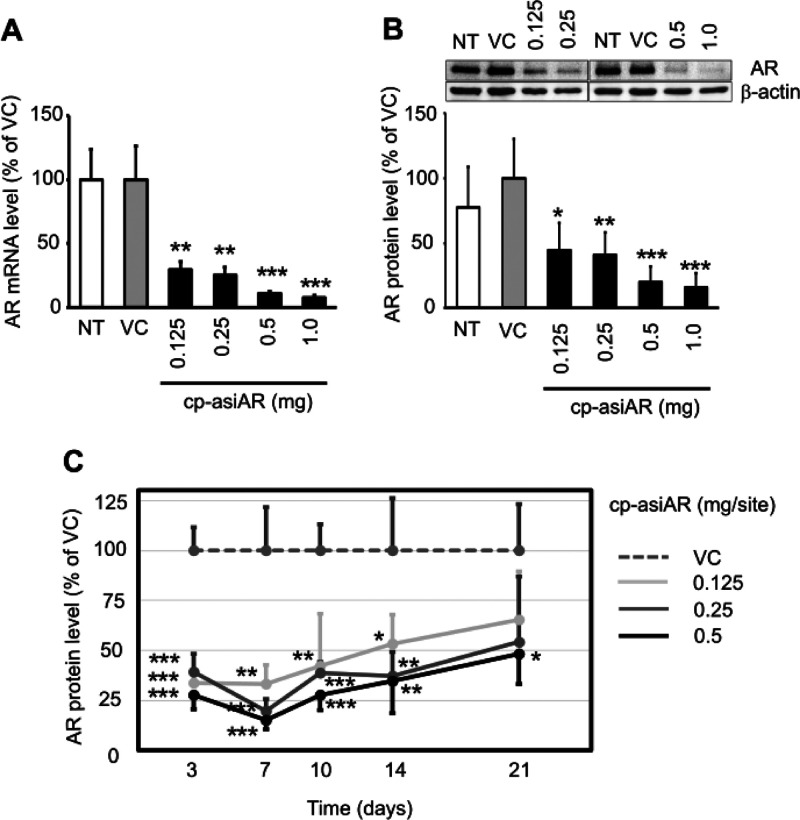
Figure 2.
In vivo validation of cp-asiAR KD efficacy. cp-asiAR was intradermally injected into the dorsal skin of mice, and the mRNA (A) or protein (B) level of AR in the injection site was measured after 24 h (n = 3). Protein level was quantitated using image analysis of western blotting (B, inset). RPL32 and β-actin were used as references for the relative mRNA and protein levels of AR, respectively. (C) cp-asiAR was injected at three doses, and skin biopsy was performed at the indicated times after injection. AR protein level normalized by β-actin was measured in the biopsy tissue. AR protein levels relative to the vehicle control are shown with the standard deviations (n = 4). Statistical significance was calculated using the t-test with the vehicle control (VC) (*, p < 0.05; **, p < 0.01; ***, p < 0.001).