Fig. 4. Endogenous fungal communities and their relationship to invasive fungal disease.
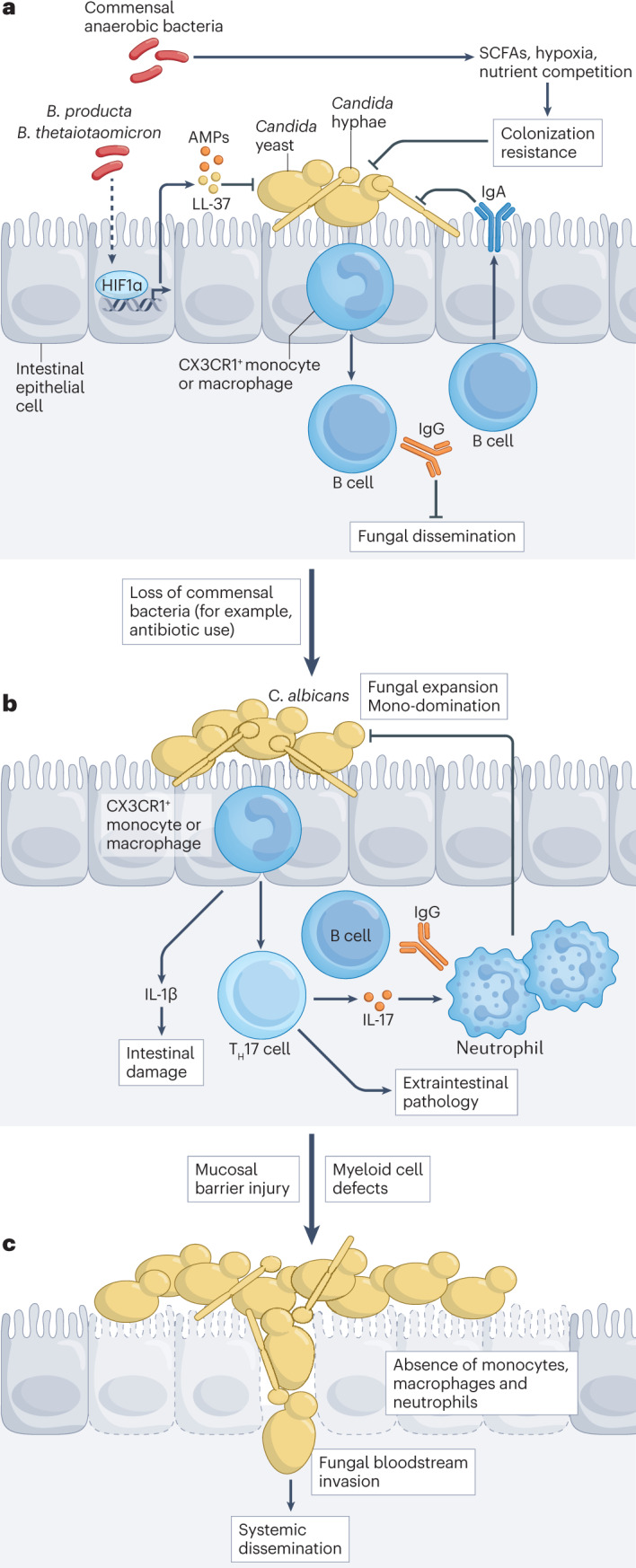
a, Mucosal and intestinal bacterial communities outnumber and compete with site-specific endogenous fungi. In the intestine, commensal anaerobic bacteria, exemplified by Blautia producta and Bacteroides thetaiotaomicron, provide colonization resistance against Candida species by activating the transcription factor hypoxia-inducible factor 1α (HIF1α) in intestinal epithelial cells to regulate the release of LL-37, an antimicrobial peptide (AMP) that restricts Candida albicans growth. Bacterial products of fermentation, specifically short-chain fatty acids (SCFAs), and metabolites likely also contribute to fungal colonization resistance and inhibition of Candida filamentation. Nutrient competition and regulation of tissue oxygen levels contribute to the balance between endogenous bacteria and competing fungal species in the gastrointestinal and reproductive tracts. CX3CR1+ mononuclear phagocytes recognize intestinal fungi through C-type lectin receptor (CLR)–CARD9 signalling and regulate the production of IgA and IgG antibodies to Candida by B cells. IgA antibodies recognize and target C. albicans adhesins (such as agglutinin-like sequence 3 (Als3)) that are primarily expressed by pseudohyphae and thus promote maintenance of the commensal yeast cell morphology. Candida-targeted IgG antibodies can protect against bloodstream invasion and disseminated candidiasis. b, In the intestinal and reproductive tracts, antibiotic-induced loss of predominantly anaerobic bacterial taxa reduces Candida colonization resistance and facilitates domination by individual fungal taxa. C. albicans strains from patients with inflammatory bowel disease with fungal dysbiosis can aggravate tissue damage, correlating with fungal strain-specific induction of IL-1β production by immune cells. The induction of T helper 17 (TH17) cells and IL-17 production can promote neutrophil recruitment to inhibit fungal tissue invasion and dissemination. However, Candida-specific TH17 cells can also promote extraintestinal inflammation. For example, mould aeroallergens can stimulate C. albicans-primed TH17 cells and aggravate fungus-associated asthma. c, Systemic bloodstream infections can arise from intestinal fungal dysbiosis with concomitant epithelial cell damage (for example, following chemotherapy) and with defects in the number or function of circulating myeloid phagocytes.
