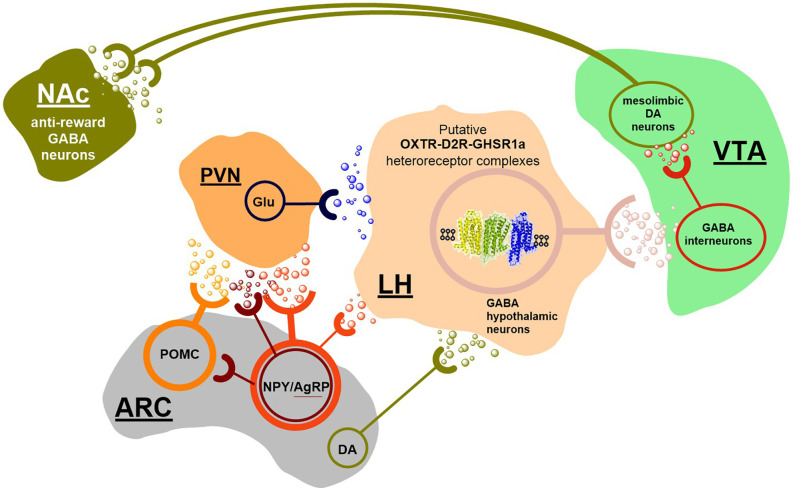
Figure 3.
Hypothesis on the brain circuitry of food reward. Food intake activates vagal afferents which reach the nucleus tractus solitarius where A2-noradrenergic positive neurons project to the hypothalamus, where the hypothalamic oxytocin neurons become stimulated with reduction of food intake and termination of the meal (not shown). However, under certain conditions the oxytocin neurons may not be the major direct target. Instead, primary targets may involve the Agouti related peptide (AgRP)/neuropeptide Y (NPY) neurons in the arcuate nucleus with orexigenic effects and the arcuate proopiomelanocortin (POMC) neurons with anorexic effects which play an important role in food intake. These two neuronal populations project to neurons in the paraventricular hypothalamic nucleus expressing melanocortin 4 receptor, almost lacking the OXTRs. There also exists a neuronal D2R in the arcuate nucleus of the hypothalamus that can contribute to the ability of the ergot D2R agonist cabergoline to inhibit food intake. To understand how the various orexigenic and anorexigenic signals in the hypothalamus, including the gastric peptide ghrelin, can be integrated into their modulation of reward circuits involving the hedonic aspects of food intake, it is proposed the existence of a GABAergic neuronal population in the lateral hypothalamus. This population can form a nucleus that can receive these signals directly or indirectly. and integrate them mainly through two types of heteroreceptor complexes, OXTR-D2R-GHS-R1a high order heterocomplexes and NMDAR-D2R heterocomplexes. This Integration process can have a major role in determining the activity of these inhibitory GABA hypothalamic neurons. These key integrative GABA neurons send projections to the GABA interneurons inhibiting DA cell groups in the ventral tegmental area belonging to the meso-limbic DA reward neurons projecting to the nucleus accumbens. The major integrative mechanism in the key hypothalamic GABA neurons shown can be the postulated OXTR-D2R-GHSRIa high-order heteroreceptor complexes in the plasma membrane of extrasynaptic and synaptic membranes of these GABA neuron populations forming a GABA nucleus. This integrative mechanism can have a major role in controlling and modulating the glutamate drive on these GABA neurons by the ability of the activated D2R protomer to open the G protein-coupled inwardly rectifying potassium (GIRK) channels leading to hyperpolarization and reduction of the glutamate drive. The modulation of the glutamate drive can also involve NMDAR-D2R heterocomplexes. A dynamic balance between glutamate drive and D2R protomer mediated inhibition of these key GABA neurons can in this way be obtained with an appropriate firing of these inhibitory GABA neurons projecting onto the GABA interneurons in the VTA area. In this way, a suitable GABA release and correct inhibition of the meso-limbic DA reward neurons can be obtained. As a result, the GABA anti-reward neurons in the nucleus accumbens involved in food reward regulation can be properly regulated. This hypothesis will be tested in future work.