Fig. 5.
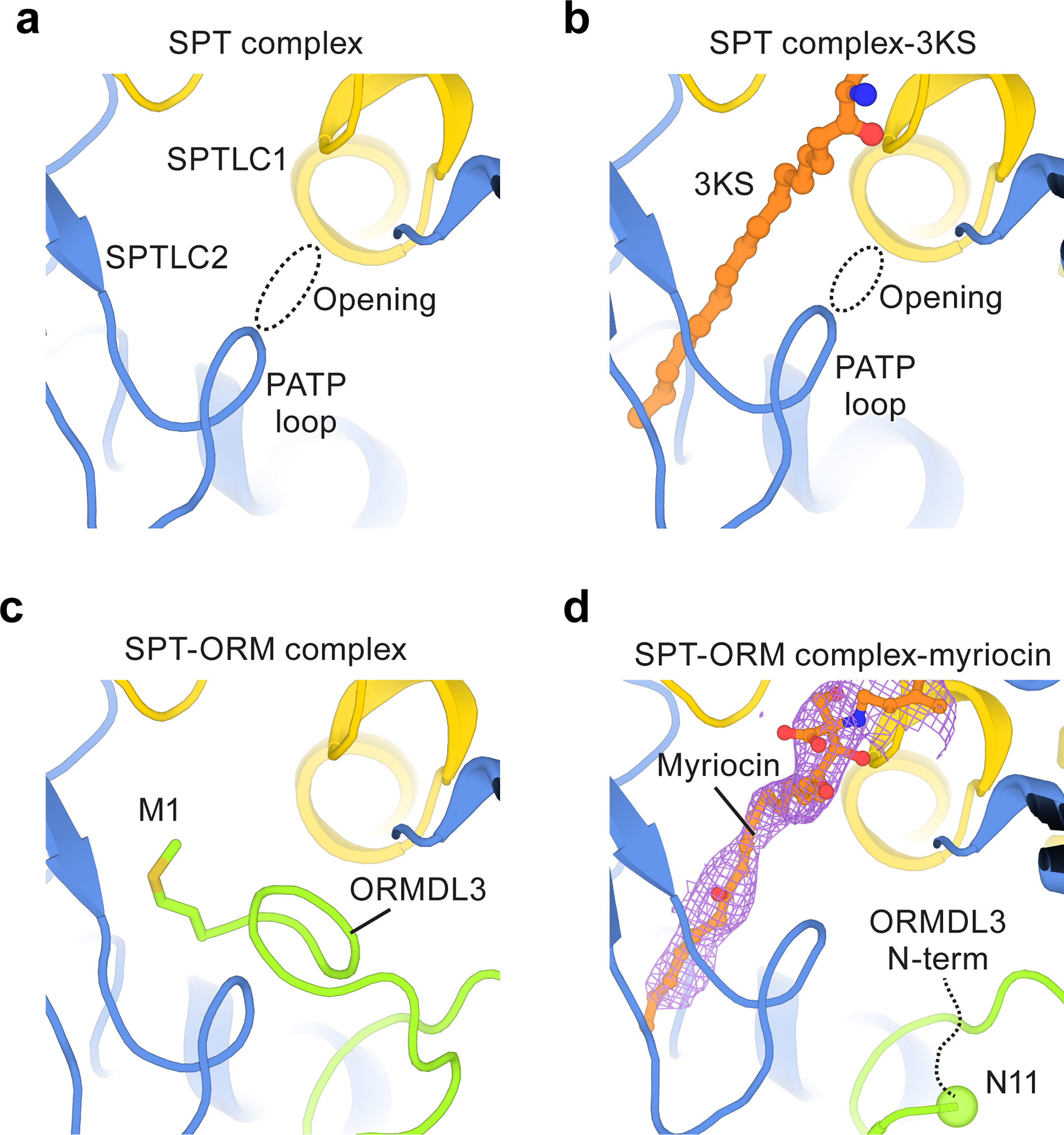
Regulation by ORMDL3.
(a) An opening that potentially allows substrate entry into the active site of the SPT complex. (b) The opening becomes narrower upon 3KS binding. (c) In the SPT-ORM complex, the N-terminus of ORMDL3 blocks the opening and occupies the substrate binding tunnel. Met1 of ORMDL3 binds to the same region as 3KS. (d) When myriocin binds to the substrate tunnel, the N-terminus of ORMDL3 rearranges. The flexible fragment (residues 1–10) of ORMDL3 is represented by a black dashed curve and Asn11 of ORMDL3 is highlighted by a sphere. The density of myriocin is shown as light pink mesh.
