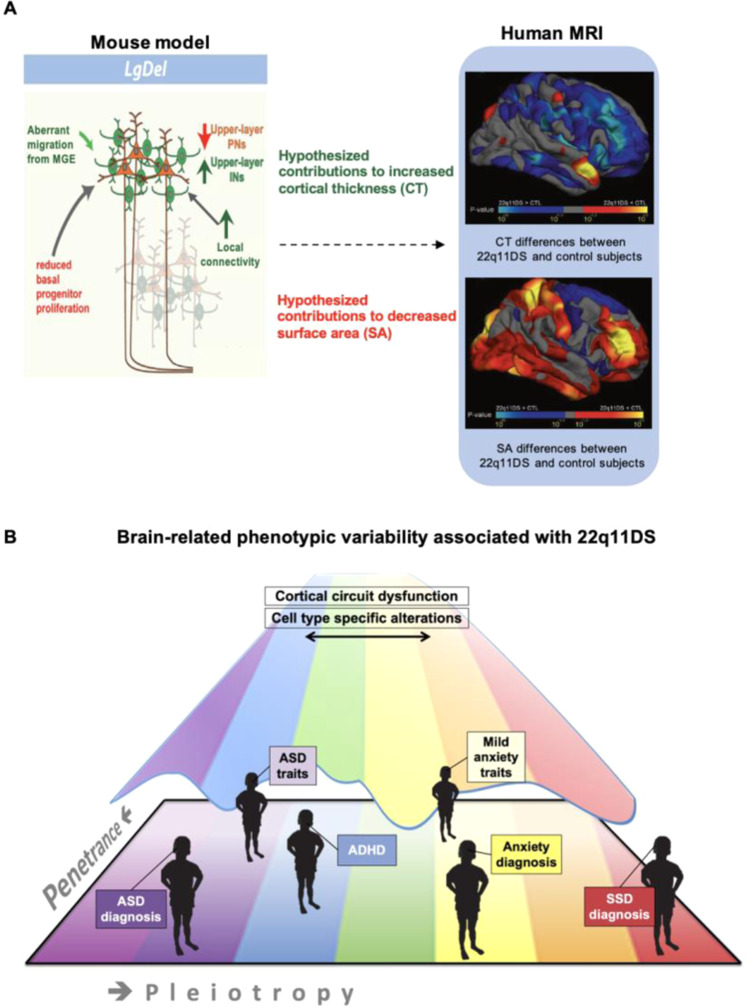
Fig. 2. Select neurobiological alterations may underlie psychiatric heterogeneity in 22q11.2 deletion syndrome.
To reduce complexity, one model animal system and imaging modality are shown to demonstrate neurobiological correlates of psychiatric phenotypes in 22q11.2 deletion syndrome. A Left panel shows a recent elegant molecular study in a 22q11.2 mouse model. Superficial (layer 2/3) pyramidal and GABA neurons are shown in focus in red-orange and green colors, respectively. These neurons had widespread alterations that were not seen in the deeper layer neurons (faded colors). Alterations in green may underlie, at least in part, cortical thickness increases in 22q11DS human subjects observed on structural MRI (right panel). Blue colors indicate greater cortical thickness (CT) in 22q11DS versus controls. Alterations in red may index, at least in part, surface area (SA) decreases in 22q11DS human subjects on structural MRI (right panel). Red colors indicate lower SA in 22q11DS versus controls. The dashed arrow indicates that these are hypothesized contributors across modalities and species, and that further studies are needed to elucidate many unanswered questions linking these associations mechanistically. B Distributed alterations in cortical structure and function in humans are hypothesized to contribute to a range of brain-related phenotypes and psychiatric disorders in 22q11DS, resulting in pleiotropy of diagnostic labels and intermediate traits, as well as a continuum of severity for each of these. ADHD attention-deficit/hyperactivity disorder, INs interneurons (GABA neurons), MGE medial ganglionic eminences, PNs projection (pyramidal) neurons, Adapted from [77, 156]).