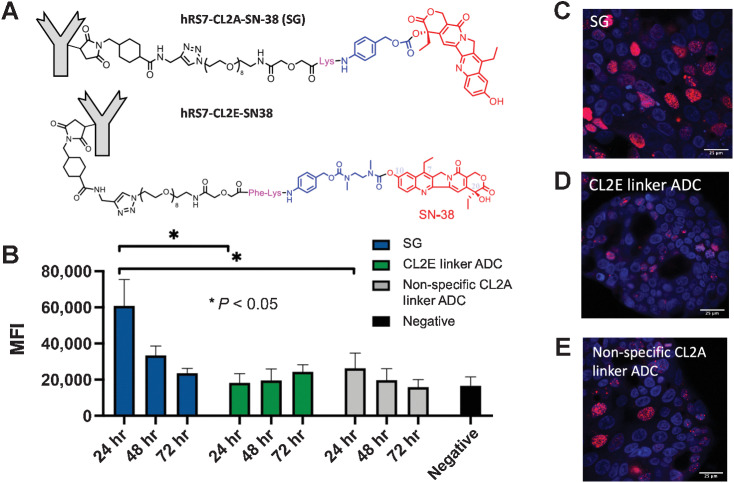
Figure 3.
In vitro Payload delivery and DNA damage. NCI-N87 cells were pulsed for 8 hours with SG (CL2A linker), the enzyme-cleavable (CL2E) linker ADC (structures shown in A), hydrolyzable (CL2A) linker nonspecific ADC, or left untreated and stained for DNA damage using γH2AX (B). Data show the median fluorescence intensity (MFI) and SD of three or four separate experiments. SG showed rapid and significant DNA damage that decreased over time, whereas the enzyme-cleavable CL2E linker ADC released the payload more slowly for lower signal that increased over 3 days. The hydrolyzable CL2A linker nonspecific control ADC showed some signal at 24 hours but lower than SG, highlighting the need for Trop-2–mediated targeting. Microscopy of NCI-N87 cells at the 24-hour time point showing nuclei (blue, Hoechst 33342) of cells treated with SG (C) with higher γH2AX signal (red) than the CL2E ADC (D) and nonspecific ADC (E).